Is Water A Element Compound Or Mixture
Juapaving
Mar 04, 2025 · 5 min read
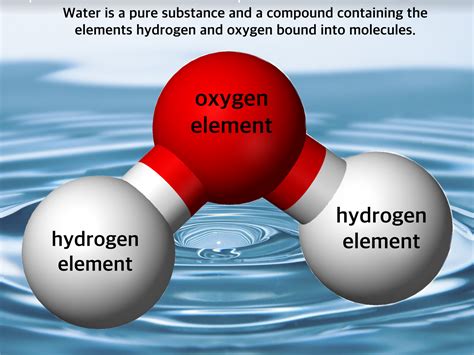
Table of Contents
Is Water an Element, Compound, or Mixture? A Deep Dive into H₂O
Water. It's the lifeblood of our planet, crucial for all known forms of life, and something we often take for granted. But have you ever stopped to consider what water actually is? Is it an element, a compound, or a mixture? The answer might seem simple, but delving deeper reveals a fascinating exploration of chemistry and the fundamental building blocks of matter. This comprehensive guide will unravel the mystery, exploring the properties of elements, compounds, and mixtures, and definitively answering the question: Is water a compound?
Understanding the Basics: Elements, Compounds, and Mixtures
Before we tackle the question directly, let's establish a firm understanding of the key terms:
Elements: The Fundamental Building Blocks
Elements are the simplest form of matter. They cannot be broken down into simpler substances by chemical means. Each element is defined by its atomic number, which represents the number of protons in its nucleus. The periodic table organizes all known elements, showcasing their properties and relationships. Examples of elements include oxygen (O), hydrogen (H), carbon (C), and iron (Fe). Elements are pure substances consisting of only one type of atom.
Compounds: Combining Elements
Compounds are formed when two or more different elements chemically combine in fixed proportions. This combination involves the sharing or transfer of electrons between atoms, creating strong chemical bonds. The properties of a compound are distinctly different from the properties of its constituent elements. For example, sodium (Na) is a highly reactive metal, and chlorine (Cl) is a toxic gas. However, when they combine, they form sodium chloride (NaCl), or table salt, a harmless and essential part of our diet. Compounds are pure substances with a fixed chemical formula.
Mixtures: A Blend of Substances
Mixtures, unlike compounds, are formed by physically combining two or more substances without chemical reactions. The components of a mixture retain their individual properties, and their proportions can vary. Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like sand and water). Mixtures are not pure substances and do not have a fixed chemical formula.
The Case of Water: H₂O
Now, let's apply our understanding to water. The chemical formula for water is H₂O, indicating that each water molecule consists of two hydrogen atoms bonded to one oxygen atom. These atoms are chemically bonded, not simply mixed together. The bond between hydrogen and oxygen is a covalent bond, where electrons are shared between the atoms. This sharing creates a stable molecule with unique properties.
This fixed chemical composition, along with the strong chemical bonds holding it together, clearly points to water being a compound, not a mixture. You cannot separate hydrogen and oxygen from water simply by physical means like filtration or evaporation. You need a chemical reaction, such as electrolysis, to break the bonds and obtain the constituent elements.
Distinguishing Water's Properties from its Constituents
The properties of water are vastly different from those of its constituent elements:
- Hydrogen (H₂): A highly flammable gas.
- Oxygen (O₂): A colorless, odorless gas essential for respiration.
- Water (H₂O): A colorless, odorless liquid essential for life, with unique properties like high surface tension, high specific heat capacity, and its ability to act as a universal solvent.
This stark difference in properties further emphasizes that water is a compound, not a mixture where the individual properties of its components would be retained.
Addressing Common Misconceptions
While the classification of water as a compound is straightforward, some misconceptions can arise:
Impurities in Water
Natural water often contains impurities like minerals, dissolved gases, and microorganisms. However, these impurities do not change the fundamental nature of water as a compound. Pure water, consisting solely of H₂O molecules, is still a compound. The impurities simply create a mixture of water and other substances. The presence of impurities in water does not negate its fundamental identity as a compound.
Different States of Water
Water exists in three states: solid (ice), liquid (water), and gas (steam). The state change (melting, freezing, boiling, condensation) is a physical change, not a chemical change. The chemical composition (H₂O) remains the same regardless of the state. Therefore, the physical state of water does not affect its classification as a compound.
Heavy Water
Heavy water (D₂O) contains deuterium (²H or D), an isotope of hydrogen with an extra neutron. While the chemical properties are similar to regular water (H₂O), the isotopic substitution does not alter the fundamental fact that it's still a compound formed from the chemical combination of two hydrogen isotopes and one oxygen atom.
The Significance of Water's Compound Nature
Understanding that water is a compound is crucial for various reasons:
- Understanding Chemical Reactions: Water participates in countless chemical reactions, both in living organisms and in industrial processes. Knowing its chemical composition and structure is vital to understanding how it interacts with other substances.
- Environmental Science: The properties of water, stemming from its compound nature, influence weather patterns, climate, and the distribution of life on Earth.
- Biological Processes: Water plays an essential role in biological processes, acting as a solvent, reactant, and medium for transport. Its chemical properties are fundamental to the functioning of living organisms.
- Industrial Applications: Water is used extensively in various industrial processes, from cooling to cleaning, and its chemical properties are critical to its effectiveness in these applications.
Conclusion: Water – A Crucial Compound
In conclusion, water (H₂O) is unequivocally a compound. Its fixed chemical composition, strong chemical bonds between hydrogen and oxygen atoms, and the distinct properties that differ significantly from its constituent elements solidify this classification. The presence of impurities or changes in physical state do not change this fact. Understanding water's fundamental nature as a compound is crucial for appreciating its importance in various scientific disciplines, from chemistry and biology to environmental science and industrial applications. It's a testament to the power of chemical bonding and the amazing complexity arising from simple elements combining to form the essential compound that sustains life on Earth.
Latest Posts
Latest Posts
-
Common Factors Of 15 And 30
Mar 05, 2025
-
How Are Photosynthesis And Cellular Respiration Connected
Mar 05, 2025
-
How To Spell The Number 12
Mar 05, 2025
-
What Are All Of The Factors Of 50
Mar 05, 2025
-
What Is 4 7 As A Percentage
Mar 05, 2025
Related Post
Thank you for visiting our website which covers about Is Water A Element Compound Or Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
