Is Sugar Dissolving In Water A Physical Or Chemical Change
Juapaving
Mar 11, 2025 · 4 min read
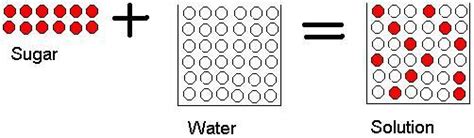
Table of Contents
Is Dissolving Sugar in Water a Physical or Chemical Change? A Deep Dive
The question of whether dissolving sugar in water is a physical or chemical change is a classic introductory chemistry conundrum. While seemingly simple, it delves into fundamental concepts of matter, its properties, and the transformations it undergoes. Understanding the difference between physical and chemical changes is crucial for grasping many scientific principles. This article will explore the dissolution of sugar in water in detail, providing a comprehensive answer and addressing common misconceptions.
Understanding Physical and Chemical Changes
Before diving into the specifics of sugar and water, let's define our key terms.
Physical Change: A physical change alters the form or appearance of a substance but doesn't change its chemical composition. The substance remains the same; only its physical properties (like shape, size, or state) are modified. Examples include melting ice, crushing a can, or dissolving salt in water (note the distinction with sugar, which we'll explore). Physical changes are often reversible.
Chemical Change: A chemical change, also known as a chemical reaction, involves the rearrangement of atoms and molecules to form new substances with different properties. The original substance is transformed into something fundamentally different. Examples include burning wood, rusting iron, or cooking an egg. Chemical changes are typically irreversible.
Analyzing the Dissolution of Sugar in Water
When you dissolve sugar (sucrose, C₁₂H₂₂O₁₁) in water (H₂O), the sugar crystals appear to vanish, forming a homogenous solution. This observation leads to the question: is this a physical or chemical transformation?
The Evidence for a Physical Change:
-
No new substance is formed: The sugar molecules (C₁₂H₂₂O₁₁) remain intact. They don't react chemically with water molecules. The solution is simply a mixture of sugar and water molecules. You can recover the sugar by evaporating the water, leaving the sugar behind. This reversibility strongly suggests a physical change.
-
Sugar retains its chemical properties: The dissolved sugar still possesses its original chemical properties. For instance, it still tastes sweet, and it can still undergo reactions typical of sucrose, such as fermentation. If a chemical change occurred, these properties would likely be altered or lost.
-
Energy change is minimal: The dissolution of sugar in water involves a small energy change (it might slightly cool or warm depending on the conditions). Significant energy changes often accompany chemical reactions, indicating the breaking and forming of chemical bonds. The subtle energy change in sugar dissolution aligns with a physical process.
-
Separation of components: The components of the sugar solution can be easily separated using physical methods. Evaporation, as mentioned above, allows the recovery of the sugar. Other separation techniques, like distillation or chromatography, could also be employed, depending on the need. Chemical separation methods are usually more complex and often involve chemical reactions.
Addressing Common Misconceptions:
Some might argue that the sugar molecules are surrounded by water molecules, forming a "hydration shell" – this appears to be a new arrangement and hence a chemical change. However, this is incorrect. While the interaction between sugar and water molecules creates a hydration shell, it is a weak intermolecular force, not a chemical bond. These forces (primarily hydrogen bonds) are easily broken, and the sugar molecules remain intact. This interaction alters the physical properties (like viscosity and sweetness), but not the chemical identity of the sugar.
The Role of Intermolecular Forces
The dissolution of sugar in water is driven by intermolecular forces. These are forces of attraction between molecules, rather than the strong intramolecular forces (chemical bonds) that hold atoms together within a molecule. In this case, the polar water molecules interact with the polar hydroxyl (-OH) groups on the sucrose molecule. This interaction weakens the attractive forces between the sugar molecules, allowing them to disperse in the water and form a homogeneous solution.
These interactions are dynamic and constantly changing, but they don't involve breaking or forming chemical bonds within the sugar or water molecules themselves. The sugar molecules are merely surrounded and dispersed, not chemically altered.
Comparing Sugar Dissolution with Other Dissolutions
To further illustrate the concept, let's compare dissolving sugar in water with a genuine chemical change, such as dissolving sodium metal (Na) in water.
In the case of sodium and water, a chemical reaction occurs:
2Na(s) + 2H₂O(l) → 2NaOH(aq) + H₂(g)
Sodium reacts vigorously with water, producing sodium hydroxide (a new substance) and hydrogen gas. This is clearly a chemical change, evidenced by the generation of a gas, heat release, and the formation of a new compound. The chemical properties of sodium are drastically different from those of sodium hydroxide.
Conclusion: A Definitive Answer
The dissolution of sugar in water is unequivocally a physical change. While water molecules surround and interact with sugar molecules, no new chemical substance is formed, and the sugar's chemical composition remains unchanged. The process is reversible, the sugar retains its chemical properties, and the energy changes are minimal. Understanding this distinction is crucial for grasping the fundamental principles of chemistry and for interpreting the behavior of matter. The process is best described as a physical phenomenon governed by intermolecular forces, not chemical reactions. The seemingly simple act of dissolving sugar reveals the intricate nature of matter and its interactions at a molecular level.
Latest Posts
Latest Posts
-
Which Statement Is Not Part Of The Cell Theory
May 09, 2025
-
Distance Is A Scalar Or Vector
May 09, 2025
-
87 96 Rounded To The Nearest Tenth
May 09, 2025
-
Action Words That Start With D
May 09, 2025
-
What Is A War Guilt Clause
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Sugar Dissolving In Water A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.