Is Sodium Chloride Inorganic Or Organic
Juapaving
Mar 14, 2025 · 4 min read
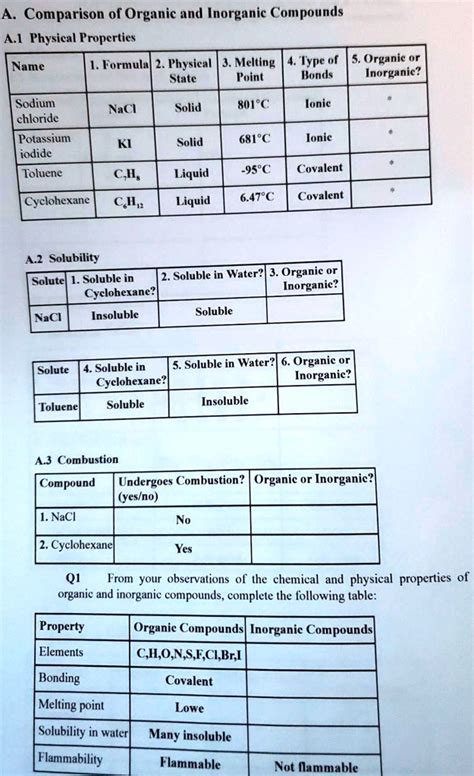
Table of Contents
Is Sodium Chloride Inorganic or Organic? A Comprehensive Look
The question, "Is sodium chloride inorganic or organic?" might seem simple at first glance. However, a deeper dive reveals the fascinating nuances of chemical classification and the very definition of "organic" and "inorganic" compounds. This article will explore the nature of sodium chloride (NaCl), commonly known as table salt, within the context of organic and inorganic chemistry, delving into the historical evolution of these classifications, and clarifying the distinctions between them.
Understanding the Definitions: Organic vs. Inorganic
The terms "organic" and "inorganic" in chemistry don't directly correlate to their everyday usage. While we often associate "organic" with natural, environmentally friendly products, in chemistry, the definition is much more precise.
Organic compounds, traditionally, were defined as compounds derived from living organisms. This definition, however, proved too restrictive as scientists began synthesizing compounds with properties identical to those found in nature. The modern definition centers around the presence of carbon atoms, specifically carbon-hydrogen (C-H) bonds. Exceptions exist (e.g., carbon dioxide, carbonates), but generally, organic compounds contain a carbon backbone with hydrogen atoms attached, often along with other elements like oxygen, nitrogen, sulfur, and halogens. These compounds usually exhibit complex structures and diverse properties. Examples include carbohydrates, proteins, lipids, and nucleic acids – the building blocks of life.
Inorganic compounds, conversely, are those that typically lack carbon-hydrogen bonds. They often consist of elements other than carbon or have simple carbon structures like carbonates or cyanides. These compounds frequently exhibit simpler structures and properties than their organic counterparts. Many inorganic compounds are found in minerals and rocks, while others are synthesized in laboratories. Examples include salts, metals, minerals, and many acids and bases.
Sodium Chloride: A Case Study in Inorganic Chemistry
Sodium chloride (NaCl), the common table salt we use daily, falls squarely into the inorganic category. Its structure is straightforward: a crystal lattice formed by the electrostatic attraction between positively charged sodium ions (Na⁺) and negatively charged chloride ions (Cl⁻). There is no carbon-hydrogen bond present in its structure. The bonding is ionic, a strong electrostatic interaction, rather than the covalent bonding prevalent in most organic compounds.
Absence of Carbon-Hydrogen Bonds: The Crucial Determinant
The absence of carbon-hydrogen bonds is the key factor distinguishing sodium chloride from organic compounds. While sodium chloride contains sodium and chlorine, both elements common in organic molecules, the way they are bonded—through ionic bonds, not covalent bonds involving carbon and hydrogen—places it firmly within the inorganic realm.
Properties Consistent with Inorganic Compounds
The properties of sodium chloride further support its inorganic classification:
- High melting and boiling points: Ionic compounds, like NaCl, have strong electrostatic forces holding them together, requiring significant energy to break these bonds and change their state.
- Crystalline structure: NaCl forms a highly ordered, crystalline structure reflecting its ionic bonding.
- Solubility in water: NaCl dissolves readily in water due to the interaction between polar water molecules and the charged ions.
- Electrical conductivity: Molten NaCl and its aqueous solution conduct electricity because the ions are free to move and carry charge.
The Evolution of Organic Chemistry: Overcoming the Vital Force Theory
The historical context of organic chemistry adds another layer to understanding the classification of sodium chloride. Early chemists believed in a "vital force," a mysterious force present only in living organisms, necessary to create organic compounds. This belief was shattered in 1828 when Friedrich Wöhler synthesized urea, an organic compound, from inorganic ammonium cyanate. This groundbreaking experiment demonstrated that organic molecules could be synthesized in a laboratory, without the need for a "vital force," fundamentally altering the definition of organic compounds and shifting the focus to the presence of carbon-hydrogen bonds.
Exceptions and Gray Areas: The Ambiguity of Definitions
While the definition of organic compounds based on carbon-hydrogen bonds provides a generally reliable framework, some exceptions and gray areas exist. Certain compounds containing carbon, such as carbon dioxide (CO₂) and carbonates (CO₃²⁻), are typically classified as inorganic despite containing carbon. This is because they lack carbon-hydrogen bonds and possess properties more aligned with inorganic compounds. They're simple in structure and don't exhibit the complex properties characteristic of most organic molecules.
Furthermore, organometallic compounds, which contain metal-carbon bonds, blur the lines between organic and inorganic chemistry. These compounds often possess characteristics of both organic and inorganic molecules. Their classification depends on the specific properties and behavior of the individual compounds.
Conclusion: Sodium Chloride Remains Firmly Inorganic
In conclusion, the question "Is sodium chloride inorganic or organic?" has a definitive answer: sodium chloride is inorganic. Its lack of carbon-hydrogen bonds, ionic bonding, and properties characteristic of inorganic compounds clearly place it within the inorganic realm. While the historical evolution of organic chemistry and the occasional exceptions to the definition might introduce a degree of complexity, the fundamental principles of chemical classification leave no room for ambiguity regarding the classification of NaCl. The absence of a carbon-hydrogen bond is the cornerstone of this classification, making sodium chloride a prime example of a simple yet crucial inorganic compound. Its ubiquitous presence in our lives underscores the importance of understanding the fundamental differences between organic and inorganic chemistry.
Latest Posts
Latest Posts
-
Midsagittal View Of The Brain With Labels
May 12, 2025
-
Force On A Current Carrying Conductor In A Magnetic Field
May 12, 2025
-
Picture Of Eukaryotic Cell And Prokaryotic
May 12, 2025
-
Metals Are Found Where On The Periodic Table
May 12, 2025
-
Data And Information Are Interchangeable Terms
May 12, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium Chloride Inorganic Or Organic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.