Is Sodium Chloride A Covalent Compound
Juapaving
Mar 18, 2025 · 5 min read
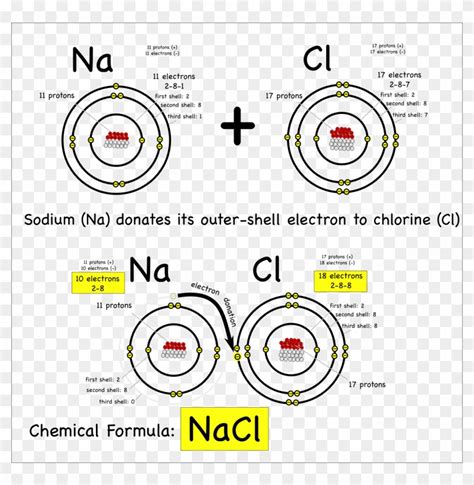
Table of Contents
Is Sodium Chloride a Covalent Compound? Understanding Chemical Bonds
The question of whether sodium chloride (NaCl), common table salt, is a covalent compound is a fundamental one in chemistry. The short answer is no, sodium chloride is not a covalent compound; it's an ionic compound. However, understanding why requires delving into the nature of chemical bonding and the properties of ionic and covalent compounds. This article will explore the differences between ionic and covalent bonds, examine the specific bonding in NaCl, and dispel any misconceptions about its covalent nature.
Understanding Chemical Bonds: The Foundation of Molecular Structure
Atoms, the basic building blocks of matter, constantly seek stability. This stability is often achieved through the formation of chemical bonds, which involve the sharing or transfer of electrons between atoms. There are two primary types of chemical bonds:
1. Covalent Bonds: Sharing is Caring
Covalent bonds arise from the sharing of electron pairs between two atoms. This sharing allows both atoms to achieve a more stable electron configuration, typically a full outer electron shell. Covalent bonds are characteristic of nonmetals bonding with other nonmetals. Examples include water (H₂O), methane (CH₄), and carbon dioxide (CO₂). The shared electrons are attracted to the nuclei of both atoms, holding them together. The strength of a covalent bond depends on the electronegativity difference between the atoms involved.
2. Ionic Bonds: Opposites Attract
Ionic bonds, on the other hand, form through the transfer of electrons from one atom to another. This transfer creates ions: positively charged cations (atoms that have lost electrons) and negatively charged anions (atoms that have gained electrons). The electrostatic attraction between these oppositely charged ions constitutes the ionic bond. Ionic bonds typically occur between metals (which tend to lose electrons) and nonmetals (which tend to gain electrons). The resulting compound is electrically neutral, as the positive and negative charges balance each other.
The Case of Sodium Chloride (NaCl): An Ionic Compound
Sodium (Na) is an alkali metal, belonging to Group 1 of the periodic table. It has one electron in its outermost shell. Chlorine (Cl) is a halogen, belonging to Group 17. It has seven electrons in its outermost shell. To achieve a stable electron configuration, sodium readily loses its single outer electron, becoming a positively charged sodium ion (Na⁺). Chlorine readily gains this electron, becoming a negatively charged chloride ion (Cl⁻).
The strong electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion forms the ionic bond in NaCl. This attraction is not a sharing of electrons, but a purely electrostatic force between opposite charges. This is the defining characteristic of an ionic compound.
Properties Supporting the Ionic Nature of NaCl
Several properties of sodium chloride strongly support its ionic nature:
-
High Melting and Boiling Points: The strong electrostatic forces between Na⁺ and Cl⁻ ions require significant energy to overcome, resulting in high melting and boiling points. Covalent compounds generally have much lower melting and boiling points.
-
Crystalline Structure: NaCl forms a regular, three-dimensional crystal lattice structure. Each Na⁺ ion is surrounded by six Cl⁻ ions, and vice versa. This ordered arrangement is a hallmark of ionic compounds. Covalent compounds often have more diverse and less ordered structures.
-
Solubility in Polar Solvents: NaCl dissolves readily in polar solvents like water (H₂O). The polar water molecules interact with the charged ions, effectively separating them and dissolving the crystal lattice. Covalent compounds, especially nonpolar ones, are often insoluble in water.
-
Electrical Conductivity: Solid NaCl does not conduct electricity because the ions are fixed in the crystal lattice. However, molten NaCl or a solution of NaCl in water conducts electricity effectively because the ions are free to move and carry charge. Covalent compounds generally do not conduct electricity.
-
Hardness and Brittleness: NaCl crystals are relatively hard but brittle. This brittleness arises from the disruption of the crystal lattice when external forces are applied, leading to repulsion between similarly charged ions. Covalent compounds exhibit a wider range of hardness.
Debunking Misconceptions: Why NaCl isn't Covalent
Some might mistakenly believe NaCl exhibits covalent character due to the presence of electrons interacting between the sodium and chlorine ions. However, this interaction is fundamentally different from the electron sharing in covalent bonds. The electrons are transferred completely, not shared. The electrostatic attraction is the dominant force holding the compound together, not the sharing of electron pairs.
Furthermore, the electronegativity difference between sodium and chlorine is very large. Electronegativity is a measure of an atom's ability to attract electrons in a bond. A large electronegativity difference strongly favors the formation of an ionic bond, as seen in the case of NaCl.
Advanced Considerations: Polarity and Partial Charges
While NaCl is primarily an ionic compound, it's crucial to acknowledge the concept of polarity. Even in predominantly ionic bonds, there can be some degree of charge distribution. While the electron transfer is largely complete, there might be a small degree of electron density remaining around the chlorine atom due to its higher electronegativity. This can lead to slight partial charges (δ+ on Na and δ- on Cl), but the overall bonding remains fundamentally ionic.
Conclusion: A Clear Case of Ionic Bonding
The overwhelming evidence points towards sodium chloride being an ionic compound, not a covalent one. The transfer of electrons, the resulting electrostatic attraction between ions, and the characteristic properties of NaCl all strongly support this classification. Understanding the fundamental differences between ionic and covalent bonds is crucial for comprehending the properties and behavior of various chemical substances. While nuances like partial charges exist, the primary bonding mechanism in NaCl is unequivocally ionic, solidifying its position as a classic example of an ionic compound and debunking any notions of it being covalent. This knowledge forms a cornerstone of chemistry, facilitating further explorations into more complex chemical interactions and phenomena.
Latest Posts
Latest Posts
-
Lcm Of 2 5 And 6
Mar 18, 2025
-
Is The Sum Of Two Rational Numbers Always Rational
Mar 18, 2025
-
What Are All The Factors Of 75
Mar 18, 2025
-
Is A Colloid Homogeneous Or Heterogeneous
Mar 18, 2025
-
What Cell Stores Food And Water
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Sodium Chloride A Covalent Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
