Is Salt A Mixture Or A Compound
Juapaving
Mar 19, 2025 · 5 min read
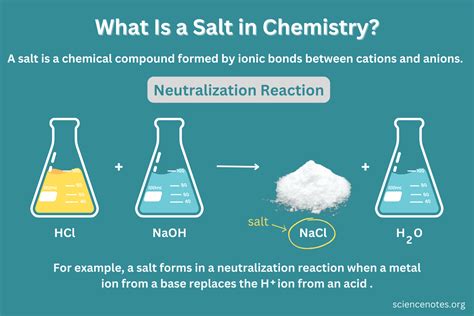
Table of Contents
Is Salt a Mixture or a Compound? Understanding the Nature of NaCl
The seemingly simple question, "Is salt a mixture or a compound?" delves into the fundamental principles of chemistry. While seemingly straightforward, the answer requires a deeper understanding of chemical bonding, molecular structure, and the properties of matter. This comprehensive article will explore the definition of mixtures and compounds, analyze the composition of salt (sodium chloride, NaCl), and definitively answer the question, providing you with a solid grasp of the underlying chemical concepts.
Defining Mixtures and Compounds
Before diving into the specifics of salt, let's establish clear definitions for mixtures and compounds. These two categories represent distinct ways in which substances can combine.
Mixtures: A Blend of Substances
A mixture is a substance composed of two or more components that are not chemically bonded. The components retain their individual chemical properties and can be separated using physical methods, such as filtration, distillation, or evaporation. Mixtures can be homogeneous (uniform in composition, like saltwater) or heterogeneous (non-uniform, like sand and water). Crucially, the properties of a mixture are often an average of the properties of its components.
Examples of Mixtures:
- Air: A mixture of nitrogen, oxygen, carbon dioxide, and other gases.
- Seawater: A mixture of water, salt, and various other dissolved minerals.
- Salad: A heterogeneous mixture of vegetables and dressing.
Compounds: Chemically Bonded Substances
A compound, in contrast, is a substance formed when two or more chemical elements are chemically bonded together. This bonding involves the sharing or transfer of electrons, resulting in a new substance with entirely different properties from its constituent elements. Compounds can only be separated into their constituent elements through chemical means, such as electrolysis or chemical reactions. The properties of a compound are distinctly different from the properties of its constituent elements.
Examples of Compounds:
- Water (H₂O): Formed from the chemical bonding of hydrogen and oxygen atoms.
- Carbon Dioxide (CO₂): Formed from the chemical bonding of carbon and oxygen atoms.
- Sodium Chloride (NaCl): Formed from the chemical bonding of sodium and chlorine atoms.
The Composition of Salt (NaCl)
Now, let's turn our attention to salt, specifically sodium chloride (NaCl). Common table salt is primarily composed of NaCl, with trace amounts of other minerals. To understand whether it's a mixture or a compound, we need to examine its formation and properties.
Sodium (Na) is a highly reactive alkali metal, while chlorine (Cl) is a highly reactive halogen. When these two elements come into contact, they undergo a vigorous chemical reaction. Sodium readily loses one electron to achieve a stable electron configuration, forming a positively charged sodium ion (Na⁺). Chlorine readily gains one electron to achieve a stable electron configuration, forming a negatively charged chloride ion (Cl⁻). The electrostatic attraction between these oppositely charged ions creates a strong ionic bond, resulting in the formation of sodium chloride, NaCl.
This ionic bond is a chemical bond, a fundamental aspect of compounds. It's not a simple physical interaction; it involves a transfer of electrons and the formation of a new substance with unique properties. NaCl has different physical and chemical properties compared to its constituent elements, sodium and chlorine. Sodium is a soft, silvery-white metal that reacts violently with water, while chlorine is a toxic, yellowish-green gas. Salt, on the other hand, is a crystalline solid that dissolves readily in water and is relatively inert.
Evidence Supporting Salt as a Compound:
- Fixed Composition: NaCl always has a fixed ratio of one sodium atom to one chlorine atom. This consistent ratio is a hallmark of compounds. Mixtures, in contrast, can have varying compositions.
- Distinct Properties: The properties of NaCl are dramatically different from those of sodium and chlorine. This difference in properties is a strong indicator of a chemical bond.
- Chemical Reactions Required for Separation: Separating sodium and chlorine from NaCl requires chemical reactions, not simply physical separation techniques. Electrolysis, for example, can be used to separate the elements.
- Crystalline Structure: The crystalline structure of salt reflects the regular arrangement of Na⁺ and Cl⁻ ions in a lattice, further emphasizing the ordered chemical bonding.
Why Salt is NOT a Mixture
The arguments against salt being a mixture are compelling. The key reason is the presence of strong chemical bonds between the sodium and chlorine atoms. In a mixture, the components are simply physically combined, retaining their individual properties. In salt, the sodium and chlorine atoms have lost their individual properties and have formed a new substance with entirely new characteristics. No physical process can separate sodium and chlorine from NaCl; only chemical processes can achieve this. Therefore, the argument that salt is a mixture due to the presence of multiple elements is fundamentally flawed.
Common Misconceptions about Salt
There are some common misconceptions surrounding the nature of salt:
- Saltwater is a mixture, not a compound: This is true. Saltwater is a homogeneous mixture of salt (NaCl, a compound) dissolved in water (H₂O, a compound). The salt and water molecules are not chemically bonded in saltwater.
- Iodized salt is a mixture: Iodized salt is a mixture because iodine (I₂) is added to NaCl. The iodine is physically mixed with the salt, not chemically bonded to it. The iodine is added to prevent iodine deficiency.
- Sea salt is a mixture: Sea salt, while primarily composed of NaCl, contains various other minerals and impurities. Therefore, sea salt is considered a mixture, containing NaCl (a compound) along with other substances.
Conclusion: Salt is a Compound
Based on the compelling evidence presented, the conclusive answer is that salt (NaCl) is a compound, not a mixture. The strong ionic bonds between sodium and chlorine atoms, the fixed composition, the distinct properties, and the requirement of chemical reactions for separation all definitively classify sodium chloride as a compound. While variations like iodized salt and sea salt can be mixtures due to the presence of additional substances, pure sodium chloride, the primary component of table salt, is unequivocally a chemical compound. Understanding this distinction highlights the fundamental differences between mixtures and compounds, laying a solid foundation for understanding more complex chemical concepts.
Latest Posts
Latest Posts
-
What Is The Complement Of A 45 Degree Angle
Mar 19, 2025
-
What Is The Secondary Structure Of Dna
Mar 19, 2025
-
What Is The Multiples Of 40
Mar 19, 2025
-
Least Common Multiple Of 60 And 24
Mar 19, 2025
-
How Is Frequency And Amplitude Related
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Is Salt A Mixture Or A Compound . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
