Is Photosynthesis An Endergonic Or Exergonic Reaction
Juapaving
Mar 16, 2025 · 5 min read
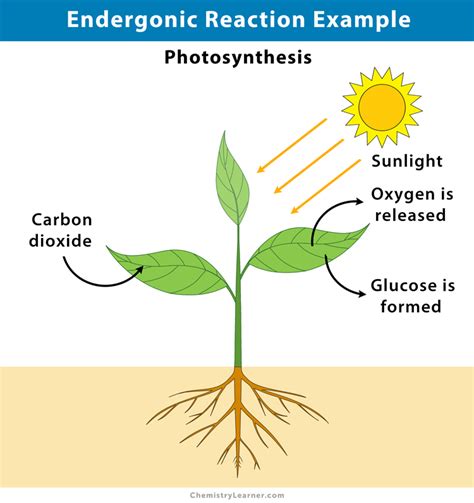
Table of Contents
- Is Photosynthesis An Endergonic Or Exergonic Reaction
- Table of Contents
- Is Photosynthesis an Endergonic or Exergonic Reaction? Understanding Energy Flow in Plants
- Defining Endergonic and Exergonic Reactions
- The Two Stages of Photosynthesis: A Closer Look
- 1. The Light-Dependent Reactions
- 2. The Light-Independent Reactions (Calvin Cycle)
- Photosynthesis: An Overall Endergonic Process
- The Role of Sunlight: The Ultimate Energy Source
- The Significance of Photosynthesis in the Ecosystem
- Understanding Photosynthesis: Implications and Applications
- Conclusion: Photosynthesis – A Vital Endergonic Process
- Latest Posts
- Latest Posts
- Related Post
Is Photosynthesis an Endergonic or Exergonic Reaction? Understanding Energy Flow in Plants
Photosynthesis, the remarkable process by which plants convert light energy into chemical energy, is a cornerstone of life on Earth. But is this intricate process an endergonic or exergonic reaction? Understanding this fundamental aspect is key to grasping the mechanics of photosynthesis and its vital role in the global ecosystem. This comprehensive article will delve into the complexities of photosynthesis, examining its energy requirements and the classification of its reactions. We'll explore the concepts of endergonic and exergonic reactions, dissect the different stages of photosynthesis, and finally, definitively answer the question: Is photosynthesis endergonic or exergonic?
Defining Endergonic and Exergonic Reactions
Before we embark on our journey into the photosynthetic process, let's establish a clear understanding of the terms "endergonic" and "exergonic." These terms describe the energy changes that occur during a chemical reaction.
Exergonic reactions release energy to their surroundings. Think of burning wood – the chemical bonds in the wood are broken, releasing energy in the form of heat and light. The products of an exergonic reaction have less free energy than the reactants. The change in Gibbs free energy (ΔG) for an exergonic reaction is negative.
Endergonic reactions, on the other hand, require an input of energy to proceed. The products of an endergonic reaction have more free energy than the reactants. The change in Gibbs free energy (ΔG) for an endergonic reaction is positive. Think of charging a battery – you need to put energy into the battery to store it.
The Two Stages of Photosynthesis: A Closer Look
Photosynthesis is not a single reaction but a complex series of reactions divided into two main stages:
1. The Light-Dependent Reactions
The light-dependent reactions occur in the thylakoid membranes within chloroplasts. These reactions are directly driven by light energy. Light photons are absorbed by chlorophyll and other pigments, exciting electrons to a higher energy level. This energy is then used to:
- Split water molecules (photolysis): This process releases electrons, protons (H+), and oxygen (O2). The oxygen is a byproduct, released into the atmosphere. This step requires energy input.
- Generate ATP (adenosine triphosphate): ATP is the cell's primary energy currency. The light-dependent reactions utilize the energized electrons to generate a proton gradient across the thylakoid membrane. This gradient drives ATP synthase, an enzyme that produces ATP through chemiosmosis.
- Produce NADPH (nicotinamide adenine dinucleotide phosphate): NADPH is a reducing agent, carrying high-energy electrons that will be used in the next stage of photosynthesis.
Energy Considerations in the Light-Dependent Reactions:
The light-dependent reactions are endergonic. They require the input of light energy to drive the splitting of water, the generation of ATP, and the production of NADPH. The energy from light is stored in the chemical bonds of ATP and NADPH.
2. The Light-Independent Reactions (Calvin Cycle)
The light-independent reactions, also known as the Calvin cycle, take place in the stroma of the chloroplast. These reactions do not directly require light but utilize the ATP and NADPH produced during the light-dependent reactions. The Calvin cycle involves a series of enzyme-catalyzed reactions that ultimately fix carbon dioxide (CO2) from the atmosphere into organic molecules, specifically glucose.
The key steps of the Calvin cycle include:
- Carbon fixation: CO2 is incorporated into a five-carbon molecule called RuBP (ribulose-1,5-bisphosphate).
- Reduction: The resulting six-carbon molecule is broken down, and the energy from ATP and NADPH is used to reduce it to form glyceraldehyde-3-phosphate (G3P), a three-carbon sugar.
- Regeneration: Some G3P is used to regenerate RuBP, ensuring the cycle continues. Other G3P molecules are used to synthesize glucose and other organic molecules.
Energy Considerations in the Light-Independent Reactions:
The light-independent reactions are also endergonic. They consume the ATP and NADPH generated during the light-dependent reactions to drive the reduction of CO2 into sugars. The energy stored in ATP and NADPH is transferred to the chemical bonds of glucose.
Photosynthesis: An Overall Endergonic Process
While both the light-dependent and light-independent reactions are individually endergonic, it's crucial to consider the overall process. Photosynthesis, as a whole, is undeniably endergonic. The net reaction involves the conversion of low-energy reactants (CO2 and H2O) into high-energy products (glucose and O2). This conversion requires a substantial input of energy, ultimately derived from sunlight.
The light-dependent reactions capture light energy and convert it into the chemical energy stored in ATP and NADPH. The light-independent reactions then utilize this stored energy to build glucose, a high-energy molecule. The overall change in Gibbs free energy (ΔG) for photosynthesis is positive, confirming its endergonic nature.
The Role of Sunlight: The Ultimate Energy Source
Sunlight serves as the primary energy source driving the endergonic nature of photosynthesis. The photons of light possess energy that is absorbed by chlorophyll and other pigments. This absorbed energy initiates a chain of events, ultimately resulting in the synthesis of glucose and the release of oxygen. Without sunlight, photosynthesis would cease.
The Significance of Photosynthesis in the Ecosystem
Photosynthesis is the foundation of most food chains on Earth. It is responsible for the conversion of inorganic carbon (CO2) into organic carbon (sugars), which are then used by plants and other organisms for growth and energy. Photosynthesis also releases oxygen, essential for aerobic respiration in most living organisms. The process plays a crucial role in regulating the Earth's atmosphere, maintaining the balance of oxygen and carbon dioxide.
Understanding Photosynthesis: Implications and Applications
Understanding the endergonic nature of photosynthesis has significant implications across various scientific fields. It informs our understanding of plant physiology, ecological processes, and the development of sustainable biofuel technologies. Research into improving photosynthetic efficiency could revolutionize agriculture and contribute to mitigating climate change.
Conclusion: Photosynthesis – A Vital Endergonic Process
In conclusion, while the individual stages of photosynthesis involve intricate energy transformations, the overall process is unequivocally endergonic. It necessitates a significant input of light energy to convert low-energy inorganic molecules into high-energy organic molecules. This endergonic nature is fundamental to the role of photosynthesis as the engine of life on Earth, fueling ecosystems and shaping our planet's atmosphere. The continuous research into this remarkable process promises to unlock further insights into its complexities and potential applications for a sustainable future.
Latest Posts
Latest Posts
-
The Storage Form Of Carbohydrates In Plants Is
Mar 18, 2025
-
Inspiratory And Expiratory Centers Are Located In The
Mar 18, 2025
-
How Many Feet Are In 300 Yards
Mar 18, 2025
-
The Storage Form Of Glucose In Plants Is
Mar 18, 2025
-
What Is The Chemical Name For Milk
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Photosynthesis An Endergonic Or Exergonic Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
