Is Phosphorus A Metal Or Nonmetal
Juapaving
Apr 07, 2025 · 5 min read
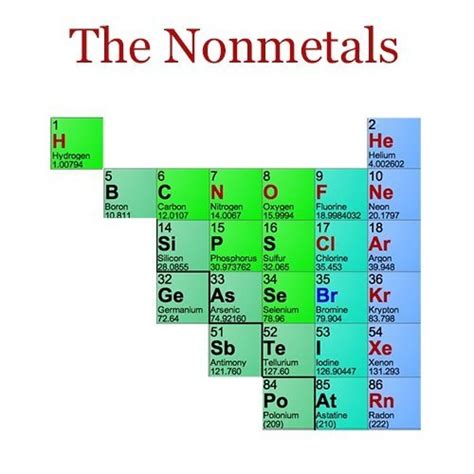
Table of Contents
Is Phosphorus a Metal or Nonmetal? Delving into the Properties of a Vital Element
Phosphorus, a fascinating and crucial element for life as we know it, often sparks the question: is it a metal or a nonmetal? The answer isn't a simple yes or no, but rather a deeper exploration into its unique properties and behavior. While definitively classified as a nonmetal, phosphorus exhibits some characteristics that blur the lines, making it a compelling subject for study. This comprehensive article will delve into the intricacies of phosphorus, examining its physical and chemical properties, its allotropes, and its crucial role in biology and industry.
Understanding the Metal vs. Nonmetal Dichotomy
Before we classify phosphorus, let's establish the fundamental differences between metals and nonmetals. Metals are typically characterized by:
- High electrical and thermal conductivity: They efficiently conduct electricity and heat.
- Malleability and ductility: They can be hammered into sheets (malleable) and drawn into wires (ductile).
- Metallic luster: They possess a shiny appearance.
- High density: They are relatively heavy for their size.
- Low electronegativity: They tend to lose electrons easily.
Nonmetals, conversely, generally exhibit:
- Poor electrical and thermal conductivity: They are insulators.
- Brittleness: They are fragile and break easily.
- Lack of metallic luster: They lack the characteristic shine of metals.
- Low density: They are relatively light.
- High electronegativity: They tend to gain electrons easily.
Phosphorus: A Nonmetal with Nuances
Phosphorus sits firmly in the nonmetal category on the periodic table. However, its behavior showcases some interesting complexities. Its position in Group 15 (also known as Group VA), alongside nitrogen, arsenic, antimony, and bismuth, indicates its nonmetallic nature. However, some of its forms display properties that might initially seem contradictory.
Allotropes: The Many Faces of Phosphorus
One of the key reasons phosphorus defies simplistic categorization is its existence in various allotropic forms. Allotropes are different structural modifications of the same element. Phosphorus exhibits several key allotropes, each with distinct properties:
-
White Phosphorus: This is the most reactive and unstable allotrope. It's a waxy, white solid that glows in the dark (phosphoresces) due to its reaction with oxygen. White phosphorus is highly toxic and ignites spontaneously in air, making it extremely dangerous to handle. Its reactivity stems from the relatively weak P-P bonds in its tetrahedral P₄ molecule.
-
Red Phosphorus: This allotrope is significantly less reactive than white phosphorus. It's a reddish-brown powder that is relatively stable in air and non-toxic. Red phosphorus is formed by heating white phosphorus, breaking down the P₄ tetrahedra into a polymeric structure. This structural change reduces reactivity.
-
Black Phosphorus: This allotrope is the least reactive and most stable form of phosphorus. It resembles graphite in appearance and structure, exhibiting a layered structure. Black phosphorus is a semiconductor, a property that bridges the gap between metals and nonmetals. This demonstrates the nuanced behavior of phosphorus.
Physical and Chemical Properties of Phosphorus: A Nonmetal Profile
Let's examine the properties that definitively classify phosphorus as a nonmetal:
- Poor Electrical Conductivity: Phosphorus is a poor conductor of electricity, a defining characteristic of nonmetals.
- Poor Thermal Conductivity: It's also a poor conductor of heat.
- Brittle Nature: Most allotropes are brittle and easily shattered.
- Low Density: Compared to metals, phosphorus has a relatively low density.
- High Electronegativity: Phosphorus has a relatively high electronegativity, indicating its tendency to attract electrons in chemical bonds. This is a hallmark of nonmetals.
- Non-Malleable and Non-Ductile: It cannot be easily shaped into sheets or wires.
The Role of Phosphorus in Biology and Industry
Phosphorus's nonmetallic characteristics play a critical role in its biological and industrial applications.
Biological Significance: The Backbone of Life
Phosphorus is an essential element for all living organisms. It's a crucial component of:
- DNA and RNA: The genetic material of all life forms utilizes phosphorus in its backbone structure. The phosphodiester bonds linking nucleotides are vital for the integrity of DNA and RNA.
- ATP (Adenosine Triphosphate): This molecule is the primary energy currency of cells. The high-energy phosphate bonds in ATP power countless cellular processes.
- Phospholipids: These are essential components of cell membranes, contributing to their structure and function.
- Bones and Teeth: Phosphorus, combined with calcium, forms the structural framework of bones and teeth in vertebrates.
Industrial Applications: From Fertilizers to Fire Retardants
The unique properties of phosphorus and its compounds have led to widespread industrial use:
- Fertilizers: Phosphorus is a vital nutrient for plant growth, and phosphate-containing fertilizers are essential for modern agriculture. This high demand highlights the importance of phosphorus in global food production.
- Detergents: Phosphates were widely used in detergents, but their contribution to water pollution led to restrictions in many areas.
- Fire Retardants: Certain phosphorus compounds act as effective fire retardants, reducing the flammability of materials.
- Matches: Red phosphorus is a key component in the striking surface of safety matches.
- Metal Alloys: Some phosphorus compounds are used in metal alloys to enhance their properties.
Conclusion: A Definitive Nonmetal with Unique Characteristics
While some properties of phosphorus, particularly its allotropic forms and the semiconducting nature of black phosphorus, might initially suggest some metallic characteristics, a comprehensive analysis firmly places phosphorus in the nonmetal category. Its poor electrical and thermal conductivity, brittle nature, high electronegativity, and its crucial role in biological systems through phosphate bonds clearly demonstrate its nonmetallic behavior. The diverse allotropes and their varying reactivities simply highlight the complex and fascinating nature of this vital element. The importance of phosphorus in biology, agriculture, and industry underlines its significance in the world around us, solidifying its status as a truly remarkable nonmetal. Further research into the unique properties of phosphorus, especially concerning its allotropic forms and their potential applications in emerging technologies, promises to continue expanding our understanding of this indispensable element. Its contributions to life and industry are undeniable, making it a worthy subject of continued scientific investigation.
Latest Posts
Latest Posts
-
Concrete Is A Pure Substance Or Mixture
Apr 09, 2025
-
What Is The North Pole Latitude
Apr 09, 2025
-
Is An Amoeba Prokaryotic Or Eukaryotic
Apr 09, 2025
-
Common Multiples Of 3 4 And 5
Apr 09, 2025
-
Is Life Science The Same As Biology
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Is Phosphorus A Metal Or Nonmetal . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.