Is Nh4br An Acid Or Base
Juapaving
Mar 13, 2025 · 5 min read
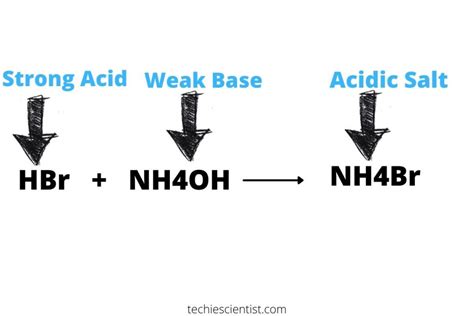
Table of Contents
Is NH₄Br an Acid or a Base? Understanding Ammonium Bromide's Properties
Ammonium bromide (NH₄Br) is a fascinating chemical compound that often sparks confusion regarding its acidic or basic nature. While it might seem straightforward, understanding its behavior requires delving into the concepts of acid-base chemistry, specifically the role of conjugate acid-base pairs and the impact of hydrolysis. This comprehensive guide will explore the properties of NH₄Br, explaining why it's considered acidic and detailing the chemical processes that lead to this classification.
Understanding Acid-Base Theories
Before diving into the specifics of NH₄Br, let's review some fundamental concepts in acid-base chemistry. Several theories exist to define acids and bases, but the most relevant for understanding NH₄Br are the Brønsted-Lowry theory and the concept of hydrolysis.
Brønsted-Lowry Theory
The Brønsted-Lowry theory defines an acid as a proton (H⁺) donor and a base as a proton acceptor. When an acid donates a proton, it forms its conjugate base, and when a base accepts a proton, it forms its conjugate acid. This conjugate pair is crucial in understanding the behavior of salts like NH₄Br.
Hydrolysis: The Key to Understanding NH₄Br's Acidity
Hydrolysis is the reaction of a substance with water. In the context of salts, hydrolysis involves the interaction of the ions of the salt with water molecules. This is where the acidic or basic nature of NH₄Br becomes apparent.
The Composition of Ammonium Bromide (NH₄Br)
Ammonium bromide is a salt formed from the reaction of a weak base (ammonia, NH₃) and a strong acid (hydrobromic acid, HBr). This is a crucial piece of information in determining its behavior in solution. Let's break down the ions involved:
-
NH₄⁺ (Ammonium ion): This is the conjugate acid of the weak base ammonia (NH₃). It has a tendency to donate a proton.
-
Br⁻ (Bromide ion): This is the conjugate base of the strong acid hydrobromic acid (HBr). It's a very weak base and doesn't significantly affect the pH of the solution.
The Hydrolysis of NH₄Br: Why it's Acidic
When NH₄Br dissolves in water, it dissociates completely into its constituent ions:
NH₄Br(s) → NH₄⁺(aq) + Br⁻(aq)
The key to understanding the acidity of NH₄Br lies in the hydrolysis of the ammonium ion (NH₄⁺):
NH₄⁺(aq) + H₂O(l) ⇌ NH₃(aq) + H₃O⁺(aq)
This equilibrium shows that the ammonium ion donates a proton (H⁺) to a water molecule, forming ammonia (NH₃) and a hydronium ion (H₃O⁺). The hydronium ion (H₃O⁺) is responsible for the increase in hydrogen ion concentration, making the solution acidic.
The Role of Conjugate Acid-Base Pairs
The strength of the conjugate acid (NH₄⁺) and the conjugate base (Br⁻) significantly impacts the pH of the solution. Since NH₃ is a weak base, its conjugate acid, NH₄⁺, is a relatively strong acid. Conversely, because HBr is a strong acid, its conjugate base, Br⁻, is extremely weak and has negligible influence on the pH.
Quantitative Analysis: Calculating the pH of NH₄Br Solution
While a precise pH calculation requires the equilibrium constant (Ka) for the ammonium ion and the initial concentration of NH₄Br, we can qualitatively determine that the solution will be acidic due to the hydronium ion production. The Ka value for NH₄⁺ is relatively small, indicating that the hydrolysis reaction is not complete, but it still produces enough H₃O⁺ to lower the pH below 7.
Comparing NH₄Br to Other Salts
To further illustrate the concept, let's compare NH₄Br to salts formed from different acid-base combinations:
-
Salt of a strong acid and strong base (e.g., NaCl): These salts produce neutral solutions because neither the cation nor the anion undergoes significant hydrolysis.
-
Salt of a weak acid and strong base (e.g., NaCH₃COO): These salts produce basic solutions because the anion undergoes hydrolysis, accepting a proton from water and generating hydroxide ions (OH⁻).
-
Salt of a strong acid and weak base (e.g., NH₄Br): These salts produce acidic solutions, as seen with NH₄Br, because the cation undergoes hydrolysis, donating a proton to water and generating hydronium ions (H₃O⁺).
Practical Applications of Ammonium Bromide
Ammonium bromide finds various applications, leveraging its unique properties:
-
Photography: Historically used in photography as a component in photographic developers and fixing agents.
-
Medicine: It has been used in some medicinal preparations, although its use has declined due to the availability of safer alternatives.
-
Fire Retardants: Its ability to release ammonia gas upon heating makes it useful in fire-retardant applications.
-
Laboratory Reagent: Commonly employed in laboratories as a reagent and in various chemical processes.
Conclusion: Understanding the Acidic Nature of NH₄Br
In summary, ammonium bromide (NH₄Br) is an acidic salt because of the hydrolysis of the ammonium ion (NH₄⁺). This hydrolysis reaction generates hydronium ions (H₃O⁺), increasing the concentration of hydrogen ions in the solution and thus lowering the pH. The extremely weak base nature of the bromide ion (Br⁻) has negligible impact. Understanding the principles of acid-base chemistry, especially the Brønsted-Lowry theory and the concept of hydrolysis, is crucial in determining the acidic or basic properties of salts like NH₄Br. The relative strengths of the conjugate acid and base derived from the parent acid and base are key to understanding the resultant pH of the salt solution. This knowledge is important in various scientific and practical applications involving NH₄Br. Further, the specific quantitative calculation of pH depends on the concentration of the NH₄Br solution and the equilibrium constant (Ka) for the ammonium ion.
Further Exploration
For a deeper understanding, explore the following topics:
- Equilibrium constants (Ka and Kb): Learn how to use these constants to calculate the pH of weak acid and weak base solutions.
- Titration curves: Understand how the pH changes during the titration of a weak acid or weak base with a strong base or strong acid.
- Buffer solutions: Investigate how buffer solutions resist changes in pH upon the addition of small amounts of acid or base.
This comprehensive exploration of NH₄Br's acidic nature aims to equip readers with a solid understanding of its properties and behavior in aqueous solutions. The application of acid-base theories, coupled with an understanding of hydrolysis, provides a framework for predicting the behavior of other salts and their solutions.
Latest Posts
Latest Posts
-
Draw A Quadrilateral That Is Not A Rhombus
May 09, 2025
-
The Cellular Organelle Responsible For Protein Synthesis Is
May 09, 2025
-
Calculate Area Of A Scalene Triangle
May 09, 2025
-
Whats The Square Root Of 484
May 09, 2025
-
Identify The Meso Isomer Of The Following Compound
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Nh4br An Acid Or Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.