Is Mixing Oil And Water A Chemical Change
Juapaving
Mar 23, 2025 · 5 min read
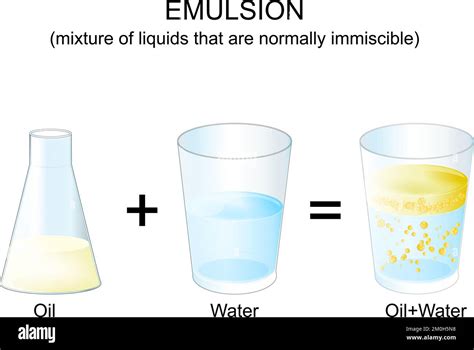
Table of Contents
- Is Mixing Oil And Water A Chemical Change
- Table of Contents
- Is Mixing Oil and Water a Chemical Change? Exploring the Science of Immiscibility
- Understanding Chemical vs. Physical Changes
- The Polarity Puzzle: Why Oil and Water Don't Mix
- The Role of Intermolecular Forces
- Exploring Misconceptions: No Chemical Reaction Occurs
- The Importance of Understanding Immiscibility
- Further Exploration: Beyond Oil and Water
- Conclusion: A Physical Phenomenon, Not a Chemical Reaction
- Latest Posts
- Latest Posts
- Related Post
Is Mixing Oil and Water a Chemical Change? Exploring the Science of Immiscibility
The seemingly simple act of mixing oil and water has captivated scientists and curious minds for centuries. The resulting distinct separation, with oil floating atop water, is a common observation. But is this separation merely a physical change, or does it represent a deeper, chemical transformation? The answer lies in understanding the fundamental properties of these substances and the nature of chemical versus physical changes. This article delves into the science behind oil and water's immiscibility, exploring why they don't mix and debunking the misconception that their interaction constitutes a chemical change.
Understanding Chemical vs. Physical Changes
Before diving into the specifics of oil and water, it's crucial to define the difference between chemical and physical changes. A physical change alters the form or appearance of a substance but doesn't change its chemical composition. Think about melting ice – it changes from a solid to a liquid, but it remains H₂O. The molecules themselves haven't changed; only their arrangement has.
A chemical change, on the other hand, involves a rearrangement of atoms and the formation of new substances with different properties. Burning wood is a prime example; the wood (cellulose) reacts with oxygen to produce ash, carbon dioxide, and water. The original substances are transformed into entirely new ones.
The Polarity Puzzle: Why Oil and Water Don't Mix
The key to understanding oil and water's incompatibility lies in their molecular structures and polarity. Water (H₂O) is a polar molecule, meaning it has a slightly positive end and a slightly negative end due to the unequal sharing of electrons between the oxygen and hydrogen atoms. This polarity allows water molecules to form strong hydrogen bonds with each other, creating a cohesive network.
Oils, on the other hand, are primarily composed of nonpolar molecules. These molecules have an even distribution of electrons, lacking the distinct positive and negative poles found in water. The absence of significant polarity prevents oils from forming strong bonds with water molecules.
This difference in polarity is the fundamental reason why oil and water don't mix. The strong hydrogen bonds within the water network repel the nonpolar oil molecules. Think of it like trying to mix magnets with different poles; the repulsive forces prevent them from blending. This phenomenon is known as immiscibility.
The Role of Intermolecular Forces
The interactions between molecules are governed by intermolecular forces. Water molecules are held together by relatively strong hydrogen bonds, while the forces between oil molecules (such as van der Waals forces) are significantly weaker. The stronger hydrogen bonds in water create a more stable and cohesive structure, effectively "pushing out" the nonpolar oil molecules.
The inability of oil and water to mix is not a result of a chemical reaction; it's a consequence of the differences in their intermolecular forces and the preference of similar molecules to associate with each other. This preference is often referred to as the principle of "like dissolves like." Polar substances dissolve in polar solvents, and nonpolar substances dissolve in nonpolar solvents.
Exploring Misconceptions: No Chemical Reaction Occurs
It's important to dispel the common misconception that mixing oil and water results in a chemical change. No new substances are formed when you combine them; the individual components retain their original chemical identities. There's no chemical reaction, no breaking or formation of chemical bonds, and no alteration of molecular structure. The separation observed is purely a physical phenomenon driven by the difference in polarity and intermolecular forces.
Some might argue that emulsification, the process of creating a temporary mixture of oil and water using an emulsifier (like soap), represents a chemical change. However, this is incorrect. Emulsifiers work by reducing the surface tension between oil and water, allowing them to form small droplets dispersed throughout each other. While the appearance changes, the chemical composition of the oil and water remains unaltered. The emulsifier itself forms a physical barrier around the oil droplets, preventing them from coalescing back into a separate layer.
The Importance of Understanding Immiscibility
Understanding the immiscibility of oil and water has significant implications across various fields:
- Environmental Science: Oil spills in aquatic environments highlight the challenge of cleaning up nonpolar pollutants from polar water bodies. The distinct separation requires specialized techniques to remediate the contamination.
- Chemistry: The concept of polarity and intermolecular forces is fundamental in chemistry, influencing solubility, reaction rates, and many other chemical processes.
- Biology: Cell membranes, composed of lipid bilayers, rely on the immiscibility of oil (lipids) and water to maintain their structure and regulate the passage of substances into and out of cells.
- Food Science: The immiscibility of oil and water plays a crucial role in the texture and stability of various food products, such as salad dressings and emulsions.
Further Exploration: Beyond Oil and Water
The principles discussed here apply beyond the simple oil-water system. The immiscibility observed is a manifestation of fundamental chemical and physical principles that govern the behavior of various substances. Many other liquid pairs exhibit similar immiscibility due to differences in polarity and intermolecular forces.
Conclusion: A Physical Phenomenon, Not a Chemical Reaction
In summary, mixing oil and water is definitively a physical change, not a chemical one. The distinct separation of these two liquids is a direct consequence of their differing molecular polarities and the resulting intermolecular forces. No new substances are formed, no chemical bonds are broken or created, and the individual components retain their original chemical identities. Understanding this fundamental principle is crucial in various scientific disciplines and has far-reaching implications in diverse applications. The seemingly simple act of mixing oil and water reveals a wealth of information about the fascinating world of molecular interactions and the principles governing the behavior of matter. The persistent separation serves as a powerful demonstration of the significance of polarity and intermolecular forces in determining the properties of matter.
Latest Posts
Latest Posts
-
X 3 1 7 15 Solve The Equation
Mar 24, 2025
-
What Weighs More A Pound Or Kilogram
Mar 24, 2025
-
What Are All The Factors Of 17
Mar 24, 2025
-
What Is The Volume Of A Solid Figure
Mar 24, 2025
-
How Did Decius Interpret Calpurnias Dream
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Is Mixing Oil And Water A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
