Is Mercury A Pure Substance Or A Mixture
Juapaving
Mar 14, 2025 · 6 min read
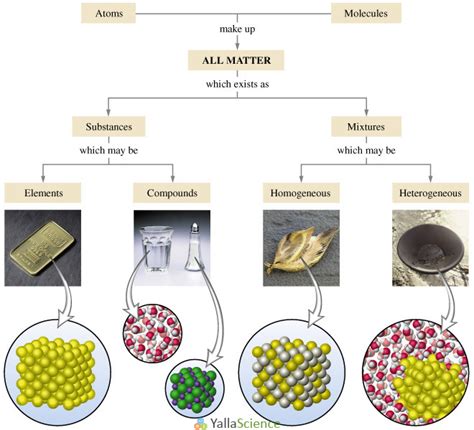
Table of Contents
Is Mercury a Pure Substance or a Mixture? A Deep Dive into Elemental Mercury
The question of whether mercury is a pure substance or a mixture often arises in chemistry discussions. The answer, while seemingly straightforward, requires a nuanced understanding of chemical definitions and the properties of mercury itself. This article will delve into the intricacies of this question, exploring the characteristics of pure substances and mixtures, examining mercury's atomic structure and behavior, and ultimately concluding with a definitive answer supported by scientific evidence.
Understanding Pure Substances and Mixtures
Before we classify mercury, let's define our terms:
Pure Substances: The Building Blocks of Matter
A pure substance is a form of matter that has a constant chemical composition and distinct chemical properties. This means that every sample of a pure substance will have the same properties regardless of its source or how it's prepared. Pure substances can be further categorized into elements and compounds:
-
Elements: Elements are fundamental substances that cannot be broken down into simpler substances by chemical means. They are composed of only one type of atom. Examples include oxygen (O), iron (Fe), and gold (Au). The periodic table organizes all known elements.
-
Compounds: Compounds are formed when two or more elements chemically combine in a fixed ratio. They have properties distinct from their constituent elements. For example, water (H₂O) is a compound formed from hydrogen and oxygen, possessing properties vastly different from its components.
Mixtures: A Blend of Substances
A mixture, on the other hand, is a combination of two or more substances that are not chemically bonded. The substances retain their individual properties, and their proportions can vary. Mixtures are further classified into:
-
Homogeneous Mixtures: In homogeneous mixtures, the components are uniformly distributed, and the mixture has a uniform composition throughout. Examples include saltwater and air.
-
Heterogeneous Mixtures: Heterogeneous mixtures have a non-uniform composition, with distinct regions of different compositions. Examples include sand and water, or a salad.
Delving into the Nature of Mercury (Hg)
Mercury, represented by the symbol Hg (from the Latin word hydrargyrum), is a heavy, silvery-white liquid metal at room temperature – a unique property among elements. Its atomic number is 80, meaning it has 80 protons in its nucleus. Let's examine key characteristics that help us classify it:
Mercury's Atomic Structure and Properties
Mercury's atomic structure explains its behavior. Its electrons are arranged in specific energy levels or shells. This electronic configuration influences its chemical bonding and reactivity. Mercury exhibits relatively weak metallic bonding compared to other metals, contributing to its liquid state at room temperature. It's also relatively unreactive, hence its historical use in thermometers and barometers.
Chemical Composition and Purity
Crucially, naturally occurring mercury consists primarily of a single isotope, Mercury-202 (²⁰²Hg), although other isotopes exist in trace amounts. This isotopic composition is nearly constant across different sources of mercury. A sample of mercury obtained from cinnabar (mercury sulfide ore) will have essentially the same chemical composition as mercury extracted from other sources. This consistency is a hallmark of a pure substance.
Separating Mercury: A Test of Purity
The fact that we can isolate mercury in its elemental form without significantly altering its chemical properties further supports its classification as a pure substance. While purification processes may remove impurities like other metals or compounds, the core chemical identity of mercury remains unchanged. We can’t separate mercury into simpler substances through chemical means; it's an element.
The Role of Impurities
It's important to acknowledge that naturally occurring mercury may contain trace amounts of impurities. These impurities, however, do not fundamentally change its nature as a pure substance. The presence of minor contaminants does not negate the fact that the vast majority of the sample is composed of mercury atoms. High-purity mercury, as used in scientific applications, undergoes rigorous purification to minimize these impurities, but even then the primary component remains elemental mercury.
Addressing Potential Misconceptions
Some might argue that mercury found in the environment is a mixture because it can exist in various forms and compounds, such as mercury sulfide (cinnabar) or methylmercury. However, these are compounds of mercury, not mercury itself. Elemental mercury remains a pure substance, irrespective of the chemical forms it might combine with.
Furthermore, even if a sample of mercury contains trace impurities, it's still predominantly elemental mercury. The classification of a substance as a mixture depends on the relative proportions of different substances and their presence in a chemically combined state. In the case of relatively pure mercury, the minor impurities don't change its primary identity as a pure substance, specifically an element.
Conclusion: Mercury – A Pure Substance
Based on our analysis of pure substances, mixtures, and the intrinsic properties of mercury, we can definitively conclude that mercury is a pure substance. Specifically, it is a chemical element consisting primarily of mercury-202 atoms, with minor isotopic variations and potentially trace impurities that do not fundamentally alter its chemical identity. While mercury can form compounds with other elements, elemental mercury itself fits the definition of a pure substance—an element in its purest form.
Further Exploration: Isotopes and Mercury's Variations
While the predominant isotope of mercury is ²⁰²Hg, other isotopes exist in smaller proportions. These isotopes are all variants of mercury, with the same number of protons (80) but differing numbers of neutrons. This isotopic variation does not disqualify mercury from being a pure substance, as all isotopes are atoms of the same element. The consistent ratio of these isotopes in naturally occurring mercury further solidifies its classification.
The Importance of Purity in Mercury Applications
The purity of mercury is crucial in various applications, from scientific research to industrial processes. Impurities can significantly alter mercury's physical and chemical properties, impacting its performance in specific applications. For instance, the presence of certain impurities in mercury used in thermometers can affect its thermal expansion coefficient, leading to inaccurate readings. Similarly, impurities in mercury used in certain industrial processes could potentially catalyze undesirable chemical reactions.
Mercury's Environmental Significance and its Forms
It is crucial to understand that while elemental mercury is a pure substance, its interaction with the environment leads to the formation of various mercury compounds. These compounds, such as methylmercury, are highly toxic and pose significant environmental and health risks. The environmental forms of mercury are mixtures, often involving organic matter and other contaminants. However, this doesn't alter the fact that elemental mercury, in its isolated form, is a pure substance.
This article aims to clarify the fundamental nature of mercury, emphasizing that elemental mercury itself is a pure substance, specifically an element, while the various forms it takes in the environment and in compounds are mixtures. A clear understanding of this distinction is crucial for both scientific accuracy and responsible environmental management.
Latest Posts
Latest Posts
-
What Is An Angle Less Than 90 Degrees
May 12, 2025
-
Name The Two Functional Groups In Amino Acids
May 12, 2025
-
What Is 5 25 In Fraction Form
May 12, 2025
-
Which Part Of The Cell Cycle Takes The Longest
May 12, 2025
-
Which Of These Has Radial Symmetry
May 12, 2025
Related Post
Thank you for visiting our website which covers about Is Mercury A Pure Substance Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.