Is Melting Wax A Physical Or Chemical Change
Juapaving
Mar 14, 2025 · 6 min read
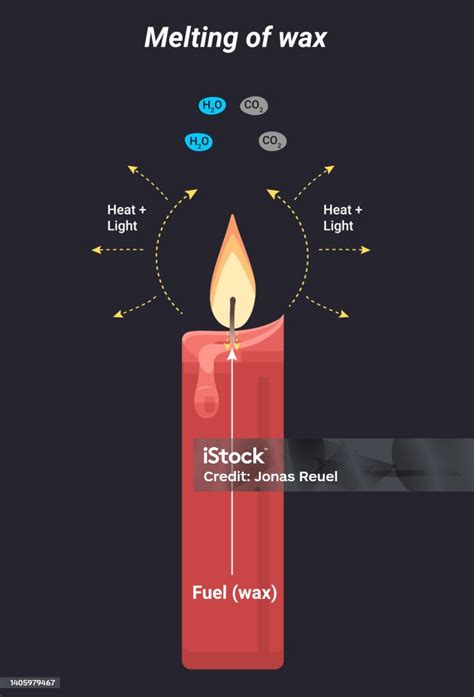
Table of Contents
Is Melting Wax a Physical or Chemical Change? A Comprehensive Exploration
The question of whether melting wax constitutes a physical or chemical change is a common one, particularly in science education. While seemingly straightforward, a complete understanding requires a deeper dive into the definitions of physical and chemical changes, and the specific properties of wax itself. This article will comprehensively explore this topic, explaining the differences between physical and chemical changes, examining the process of wax melting in detail, and debunking common misconceptions. We'll also touch upon the various types of wax and how their melting behavior might slightly differ.
Understanding Physical and Chemical Changes
Before we delve into the specifics of melting wax, it's crucial to establish a clear understanding of the fundamental differences between physical and chemical changes.
Physical Changes
A physical change alters the form or appearance of a substance but doesn't change its chemical composition. The molecules themselves remain the same; only their arrangement or state of matter changes. Examples of physical changes include:
- Changes in state: Melting, freezing, boiling, condensation, and sublimation are all physical changes. The substance remains the same; only its state (solid, liquid, gas) changes.
- Changes in shape: Cutting, bending, crushing, or breaking a substance are physical changes. The chemical makeup of the material remains unchanged.
- Dissolving: Dissolving sugar in water is a physical change. The sugar molecules are dispersed in the water, but they retain their chemical identity.
Chemical Changes
A chemical change, also known as a chemical reaction, involves the transformation of one or more substances into new substances with different chemical properties. This involves the breaking and forming of chemical bonds, resulting in a change in the molecular structure. Examples of chemical changes include:
- Burning: Combustion is a chemical change that involves the reaction of a substance with oxygen, producing heat and new substances (like carbon dioxide and water).
- Rusting: The formation of rust (iron oxide) from iron is a chemical change caused by a reaction with oxygen and water.
- Cooking: Many cooking processes involve chemical changes, such as the browning of meat (Maillard reaction) or the setting of an egg.
The Melting of Wax: A Detailed Analysis
Now, let's apply this knowledge to the melting of wax. When wax is heated, it transitions from a solid to a liquid state. This transition involves a change in the arrangement of the wax molecules. In the solid state, the molecules are closely packed and arranged in an ordered structure, giving the wax its solid form. As heat is applied, the molecules gain kinetic energy, overcoming the intermolecular forces holding them together. This allows them to move more freely, resulting in the transition to a liquid state. Crucially, the chemical composition of the wax remains unchanged. The long hydrocarbon chains that constitute the wax molecule are not broken or rearranged during melting. The only change is the state of matter and the arrangement of the molecules.
Therefore, melting wax is unequivocally a physical change.
Different Types of Wax and Their Melting Behavior
While the fundamental principle remains consistent across various wax types, subtle differences in their melting points and behavior exist due to variations in their chemical composition. Common types of wax include:
- Paraffin wax: Derived from petroleum, paraffin wax is a common type used in candles and other applications. Its melting point typically ranges from 46°C to 68°C.
- Beeswax: A natural wax produced by honeybees, beeswax has a higher melting point than paraffin wax, typically around 62°C to 65°C. It also possesses a unique chemical composition compared to paraffin wax.
- Soy wax: A vegetable-based wax made from soybeans, soy wax is gaining popularity as a more sustainable alternative to paraffin wax. Its melting point is generally lower than paraffin wax, often around 49°C to 54°C.
- Carnauba wax: Derived from the leaves of the carnauba palm tree, Carnauba wax has a high melting point, typically above 80°C.
Despite these differences in melting points and origins, the melting process for all these waxes remains a physical change. The molecules change their arrangement and state, but their chemical structure remains intact.
Debunking Common Misconceptions
Several misconceptions surround the melting of wax, often stemming from a misunderstanding of the concepts of physical and chemical changes.
Misconception 1: The color change of wax upon melting indicates a chemical change.
Reality: The change in appearance, including color and opacity, is a result of the change in the arrangement of wax molecules, affecting light scattering and absorption, not a change in the chemical composition.
Misconception 2: The emission of slight fumes during the melting of some waxes implies a chemical reaction.
Reality: While some waxes might emit slight fumes upon melting, this is often due to the evaporation of volatile impurities or low-molecular-weight components within the wax, not a fundamental change in the wax's chemical structure. This evaporation is a physical process, not a chemical one.
Misconception 3: The solidified wax after cooling is a different substance.
Reality: While the structure might have subtly changed during the cooling process, the chemical composition remains the same as the original wax. The cooling process simply reverses the melting process, returning the wax to its solid state.
Practical Applications and Further Considerations
The understanding that melting wax is a physical change has significant practical implications. For example, in candle making, the ability to repeatedly melt and solidify wax without altering its chemical properties allows for the reuse of leftover wax. This knowledge is also vital in various industrial applications where wax is used as a coating, sealant, or component in various products.
While we've primarily focused on the melting of pure wax, the presence of additives or impurities can sometimes introduce complexities. For instance, if a candle wax contains dyes or fragrances, the heating process could potentially affect these additives, leading to minor chemical changes in those specific components, although not in the primary wax itself. This however, remains a localized change, and does not fundamentally alter the nature of the primary wax melting process, which continues to be a physical change.
Conclusion
In conclusion, melting wax is unequivocally a physical change. The process involves a change in the state of matter and the arrangement of molecules, but the chemical composition of the wax remains unaltered. Understanding this fundamental distinction is crucial not only for scientific accuracy but also for numerous practical applications across various industries. While nuances exist due to wax type and the presence of additives, the core principle remains consistent: the melting of wax represents a reversible physical transformation. This understanding dispels common misconceptions and highlights the importance of precise scientific terminology in describing physical and chemical phenomena.
Latest Posts
Latest Posts
-
How Many Times Does Dna Replicate In Mitosis
May 09, 2025
-
How Many Square Feet Is 400 Square Meters
May 09, 2025
-
First 30 Elements Of Periodic Table
May 09, 2025
-
Breakdown Of Glucose To Pyruvic Acid
May 09, 2025
-
Is Crushing A Can A Physical Change
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Wax A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.