Is Melting Wax A Chemical Or Physical Change
Juapaving
Mar 10, 2025 · 6 min read
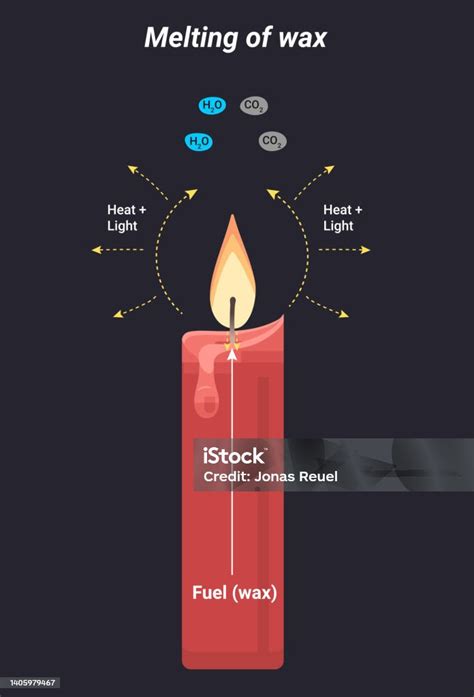
Table of Contents
Is Melting Wax a Chemical or Physical Change? A Deep Dive
Melting wax is a quintessential example used to illustrate the difference between physical and chemical changes. While seemingly simple, a thorough understanding requires exploring the fundamental concepts of matter, its states, and the transformations it undergoes. This comprehensive guide will delve into the nature of melting wax, examining the evidence supporting its classification as a physical change, while also addressing common misconceptions.
Understanding Physical and Chemical Changes
Before we dissect the melting of wax, let's establish a clear understanding of the terms "physical change" and "chemical change." These terms describe transformations matter undergoes, categorized by whether the fundamental composition of the substance alters.
Physical Changes
A physical change involves a transformation that alters the form or appearance of matter but doesn't change its chemical composition. The substance retains its original chemical identity. Examples include:
- Changes in state: Melting, freezing, boiling, condensation, sublimation (solid to gas), and deposition (gas to solid). These changes involve altering the arrangement of particles but not their fundamental nature.
- Changes in shape: Cutting, bending, crushing, grinding. These changes modify the physical form without affecting the chemical makeup.
- Dissolving: Dissolving sugar in water changes the form but doesn't alter the sugar's chemical structure. The sugar can be recovered by evaporating the water.
Key characteristics of physical changes:
- No new substance is formed. The original substance is still present, albeit in a different form.
- Changes are usually reversible. The original substance can often be recovered through physical means.
- Changes involve relatively small energy changes. The energy changes associated with physical changes are typically less dramatic than those in chemical changes.
Chemical Changes
A chemical change, also known as a chemical reaction, involves a transformation that alters the fundamental chemical composition of a substance. New substances with different properties are formed. Examples include:
- Burning: Combustion reactions involve reacting a substance with oxygen to produce new compounds (often oxides), heat, and light.
- Rusting: Iron reacting with oxygen and water to form iron oxide (rust).
- Cooking: Many cooking processes involve chemical changes, such as the browning of meat (Maillard reaction).
- Digestion: The breakdown of food in the body involves numerous chemical reactions.
Key characteristics of chemical changes:
- New substances are formed. The original substance is transformed into one or more different substances with different properties.
- Changes are usually irreversible. The original substance cannot be easily recovered through simple physical means.
- Changes often involve significant energy changes. Chemical reactions may release (exothermic) or absorb (endothermic) substantial amounts of energy.
Examining the Melting of Wax: A Physical Transformation
Now, let's apply these definitions to the melting of wax. When wax is heated, it transitions from a solid to a liquid state. This change is purely physical. Here's why:
1. No New Substance is Formed
During the melting process, the wax molecules themselves remain unchanged. The chemical bonds within the wax molecules are not broken or reformed. The only change is in the arrangement and movement of these molecules. In solid wax, the molecules are closely packed and have limited movement. Upon heating, they gain kinetic energy, overcoming the intermolecular forces holding them together, allowing them to move more freely, resulting in the liquid state. The chemical formula of the wax remains identical before and after melting.
2. The Change is Reversible
Cooling the liquid wax allows it to solidify, returning it to its original solid state. This reversibility is a hallmark of a physical change. The process can be repeated multiple times without altering the fundamental chemical composition of the wax.
3. Relatively Small Energy Change
While energy is required to melt the wax (endothermic process), the amount of energy involved is relatively small compared to the energy changes seen in chemical reactions. The energy primarily breaks intermolecular forces, not strong covalent bonds within the wax molecules.
Types of Wax and Their Melting Points
It’s important to note that different types of waxes have different melting points. This variation stems from the chemical structure of the individual wax molecules and the resulting intermolecular forces. Paraffin wax, a common type, typically melts between 46°C and 68°C (115°F and 154°F), whereas beeswax has a higher melting point, around 62°C to 65°C (144°F to 149°F). The melting point itself, while differing for various wax types, doesn't negate the fact that the melting process remains a physical change. The differences in melting point highlight the influence of molecular structure on the strength of intermolecular forces.
Addressing Common Misconceptions
Some might argue that the slight color change or odor emitted during wax melting indicates a chemical change. However, these observations are often due to:
- Impurities: Most waxes aren’t pure substances. They contain impurities that might decompose or volatilize at higher temperatures. The color or odor change is attributable to these impurities undergoing a change, not the wax itself.
- Oxidation: Exposure to air during melting can lead to slight oxidation of some wax components, causing a change in color or odor. This is a chemical reaction, but it’s a secondary reaction and doesn't constitute the primary process of melting, which is physical.
The key is to focus on the primary transformation: the change in state from solid to liquid. This change, at its core, is purely physical, unaffected by minor changes in color or odor that can arise from impurities or slow oxidation.
The Molecular Perspective
To further solidify the understanding that melting wax is a physical change, consider the molecular behavior. In solid wax, the molecules are arranged in a relatively ordered structure. As heat is applied, the molecules gain kinetic energy, causing them to vibrate more vigorously. This increased vibrational energy eventually overcomes the intermolecular forces holding the molecules together in the solid lattice. The molecules then transition to a more disordered, liquid state, where they can move more freely past one another. Crucially, the covalent bonds within the individual wax molecules remain intact throughout this process. The change is one of molecular arrangement and mobility, not a change in the chemical structure of the wax molecules themselves.
Practical Applications and Implications
Understanding that melting wax is a physical change has practical implications across various applications:
- Candle making: The ability to melt and solidify wax repeatedly is fundamental to candle making. The physical nature of the process allows for repeated melting and reshaping without altering the chemical properties of the wax.
- Wax sealing: The reversibility of wax melting makes it useful for sealing envelopes or documents. The melted wax can be solidified to create a seal which, while strong, can be removed with gentle heating.
- Industrial uses: Wax is utilized in numerous industrial processes, such as coating, waterproofing, and creating lubricants. Its ability to transition between solid and liquid states without chemical alteration is crucial for these applications.
Conclusion
In conclusion, the melting of wax is unequivocally a physical change. While minor changes in color or odor might occur due to impurities or oxidation, the primary transformation—the transition from solid to liquid—involves only a change in the physical state and arrangement of molecules, not a change in their chemical composition. The reversibility of the process, the relatively small energy changes involved, and the absence of new substance formation all strongly support this classification. A firm grasp of this concept provides a solid foundation for understanding the broader distinction between physical and chemical changes and their significance in various scientific and practical contexts. This knowledge is essential for anyone working with wax, from candle makers to industrial chemists.
Latest Posts
Latest Posts
-
How Many Times Does Dna Replicate In Mitosis
May 09, 2025
-
How Many Square Feet Is 400 Square Meters
May 09, 2025
-
First 30 Elements Of Periodic Table
May 09, 2025
-
Breakdown Of Glucose To Pyruvic Acid
May 09, 2025
-
Is Crushing A Can A Physical Change
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Wax A Chemical Or Physical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.