Is Melting Ice A Chemical Reaction
Juapaving
Mar 13, 2025 · 5 min read
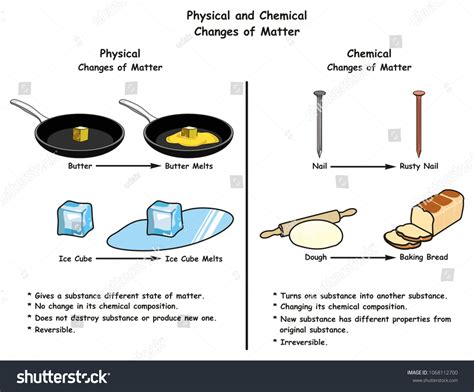
Table of Contents
Is Melting Ice a Chemical Reaction? Understanding Physical vs. Chemical Changes
The question of whether melting ice is a chemical reaction is a common one, often sparking debate among students and science enthusiasts alike. The simple answer is no, melting ice is not a chemical reaction. It's a physical change. Understanding the difference between physical and chemical changes is crucial to grasping this concept. This article will delve deep into the nature of melting ice, exploring the molecular processes involved and contrasting them with the characteristics of chemical reactions. We'll also examine common misconceptions and provide clear examples to solidify your understanding.
The Difference Between Physical and Chemical Changes
Before we dive into the specifics of melting ice, let's establish a firm understanding of the core difference between physical and chemical changes.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. The molecules remain the same; only their arrangement or state of matter changes. Examples include:
- Melting: Solid to liquid (like ice to water)
- Freezing: Liquid to solid (water to ice)
- Boiling: Liquid to gas (water to steam)
- Condensation: Gas to liquid (steam to water)
- Dissolving: A substance disperses in a solvent (sugar in water) – the sugar molecules are still sugar molecules.
- Crushing: Changing the shape of a solid (crushing a can)
Chemical changes, on the other hand, involve a transformation of one or more substances into entirely new substances with different chemical properties. This involves the breaking and forming of chemical bonds, resulting in a change in the molecular structure. Examples include:
- Burning: Combustion reactions, such as burning wood or paper.
- Rusting: Oxidation of iron in the presence of oxygen and water.
- Cooking: Many cooking processes involve chemical changes, like baking a cake.
- Digestion: The breakdown of food in our bodies.
Melting Ice: A Detailed Look at the Physical Process
When ice melts, it transitions from a solid state to a liquid state. This transformation is driven entirely by changes in kinetic energy. The molecules in ice are held together in a rigid, crystalline structure by relatively strong intermolecular forces (hydrogen bonds). At temperatures below 0°C (32°F), these forces effectively restrict the movement of water molecules, keeping them in fixed positions.
As heat is applied to the ice, the kinetic energy of the water molecules increases. This increased energy overcomes the intermolecular forces holding the molecules in place, allowing them to move more freely. The rigid structure of the ice breaks down, and the molecules transition from a highly ordered state to a more disordered, fluid state—liquid water.
Crucially, the chemical formula of water remains unchanged throughout this process. It remains H₂O. No new chemical bonds are formed, and no existing bonds are broken. The only change is the arrangement and movement of the water molecules.
Visualizing the Molecular Transformation
Imagine a neatly arranged stack of oranges (representing water molecules in ice). As you apply heat (energy), the oranges begin to jiggle and move around more. Eventually, the stack collapses, and the oranges become less organized but are still the same oranges. This is analogous to the melting of ice. The water molecules are still water molecules; their arrangement simply changes.
Addressing Common Misconceptions
Several misconceptions often surround the melting of ice, leading to confusion about its nature. Let's address some of these:
-
Misconception 1: A change in state always implies a chemical reaction. This is false. Changes in state, like melting, freezing, boiling, and condensation, are fundamentally physical changes. They only involve a rearrangement of molecules, not a change in their chemical identity.
-
Misconception 2: The appearance of water differs significantly from ice, suggesting a chemical reaction. While liquid water appears different from ice, this is simply a result of the different molecular arrangements and intermolecular forces. The chemical composition is identical.
-
Misconception 3: Energy changes imply a chemical reaction. While energy is absorbed during melting (endothermic process), energy changes are associated with both physical and chemical changes. Melting ice is an endothermic process because energy is absorbed to break the intermolecular forces holding the water molecules in the ice structure. However, this energy absorption does not constitute a chemical reaction.
Comparing Melting Ice to a Chemical Reaction
To further clarify, let's contrast melting ice with a clear example of a chemical reaction: burning wood.
When wood burns, it reacts with oxygen in the air, undergoing a combustion reaction. This reaction produces entirely new substances, including carbon dioxide, water vapor, and ash. The chemical bonds in the wood molecules are broken, and new bonds are formed to create different molecules. The chemical composition has fundamentally changed.
In contrast, melting ice involves no such transformation. The water molecules remain H₂O molecules, merely shifting from a rigid, ordered structure to a more fluid, disordered state. No new substances are formed, and no chemical bonds are broken or created.
The Importance of Understanding Physical Changes
Understanding the difference between physical and chemical changes is fundamental to numerous scientific disciplines, including chemistry, physics, and materials science. This knowledge is crucial for:
- Predicting the behavior of materials: Knowing whether a process is physical or chemical helps predict how materials will behave under different conditions.
- Designing and developing new materials: Understanding physical changes enables scientists to design materials with specific properties by altering their physical state or structure.
- Understanding environmental processes: Many natural phenomena, such as the melting of glaciers or the evaporation of water, are physical changes. Understanding these processes is crucial for environmental science and climate change studies.
Conclusion: Melting Ice – A Purely Physical Phenomenon
In conclusion, melting ice is unequivocally a physical change, not a chemical reaction. The process involves a transition of state from solid to liquid, driven by changes in the kinetic energy of water molecules. While energy is absorbed during the process, the chemical composition of the substance remains unchanged—it's still H₂O. Understanding this distinction is crucial for a robust grasp of fundamental scientific principles and their application in various fields. Misconceptions about the nature of melting ice should be clarified to foster a deeper understanding of physical and chemical transformations. By differentiating physical and chemical changes, we can more accurately interpret and predict the behavior of matter in the world around us.
Latest Posts
Latest Posts
-
The Rate At Which Energy Is Used Is Called
May 09, 2025
-
Do Nails Keep Growing After Death
May 09, 2025
-
Conserving Fuel Is Important Because Burning Fuel
May 09, 2025
-
Red Blood Cells Placed In A Hypotonic Solution Will
May 09, 2025
-
How Do You Write 1 2 As A Percentage
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Ice A Chemical Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.