Is Melting Butter Physical Or Chemical Change
Juapaving
Mar 29, 2025 · 5 min read
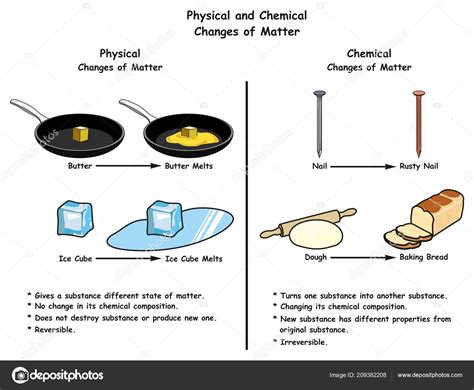
Table of Contents
Is Melting Butter a Physical or Chemical Change? A Comprehensive Look
The seemingly simple act of melting butter sparks a fascinating question in the world of chemistry: is it a physical or chemical change? Understanding the difference between these two types of changes is crucial for grasping fundamental scientific concepts. This article will delve deep into the process of melting butter, exploring its properties before and after melting, and ultimately determining its classification. We'll explore the broader context of physical and chemical changes, examining examples of each to solidify our understanding.
Understanding Physical and Chemical Changes
Before we tackle the butter conundrum, let's establish clear definitions. A physical change alters the form or appearance of a substance but doesn't change its chemical composition. The substance remains the same; only its physical properties (like shape, size, or state of matter) are modified. Think of cutting paper, dissolving sugar in water, or freezing water into ice. These are all physical changes because the fundamental chemical makeup of the paper, sugar, and water remains unchanged.
A chemical change, on the other hand, involves a transformation in the chemical composition of a substance. New substances with different properties are formed, often accompanied by observable signs like a color change, gas production, or heat release. Examples include burning wood (forming ash and gases), rusting iron (forming iron oxide), or baking a cake (complex chemical reactions transforming ingredients).
Key Differences Summarized:
| Feature | Physical Change | Chemical Change |
|---|---|---|
| Composition | Remains the same | Changes; new substances are formed |
| Properties | Only physical properties (shape, size, state) change | Chemical properties and physical properties change |
| Reversibility | Often reversible (e.g., melting ice) | Usually irreversible (e.g., burning wood) |
| Energy | Energy changes are usually small | Significant energy changes (heat released or absorbed) |
The Case of Melting Butter: A Detailed Examination
Now, let's return to our primary question: is melting butter a physical or chemical change? To answer this, we'll analyze the process step-by-step.
Butter is primarily composed of triglycerides, which are types of fats. These triglycerides are esters of fatty acids and glycerol. When we heat butter, the triglycerides absorb thermal energy. This energy increases the kinetic energy of the molecules, causing them to vibrate more vigorously and overcome the intermolecular forces holding them in a solid structure.
As the temperature rises, the butter transitions from a solid to a liquid state. This is the melting process. Crucially, the chemical bonds within the triglyceride molecules themselves remain intact. The fatty acids and glycerol are still connected in the same way. No new substances are formed. The only change is the state of matter – from solid to liquid. We can even solidify the melted butter by cooling it back down, further demonstrating the reversibility of this process.
Evidence Supporting a Physical Change:
- Reversibility: As mentioned, melted butter can be solidified by cooling, demonstrating reversibility, a hallmark of physical changes.
- No new substance formation: The chemical composition of the butter remains unchanged. The triglycerides are still present in the same form, just in a different state.
- No gas production or significant color change: There's no visible evidence of new substances forming. A slight browning might occur with prolonged high-heat exposure, but this is a separate Maillard reaction, a chemical change we will discuss separately.
- Minimal energy change (relative to chemical changes): The energy required to melt butter is relatively small compared to the energy involved in chemical reactions like combustion.
The Maillard Reaction: A Separate Chemical Change
While the melting of butter itself is a physical change, prolonged heating at high temperatures can lead to a different, chemically significant process: the Maillard reaction. This reaction occurs between amino acids and reducing sugars, producing hundreds of different flavor and aroma compounds, including melanoidins (responsible for the browning).
This browning is a clear indication of a chemical change. The original chemical compounds in the butter have reacted to form new, different substances. Therefore, while the initial melting is physical, excessive heating introduces a distinct chemical transformation.
Distinguishing Melting from the Maillard Reaction:
It's crucial to differentiate between the simple melting of butter and the Maillard reaction. Melting is a phase transition – solid to liquid – involving only a change in physical state. The Maillard reaction, however, involves the formation of entirely new chemical compounds, altering the composition of the butter. They are distinct processes occurring at different temperatures and with different consequences. Low-temperature melting keeps the chemical composition largely the same, while high-temperature exposure initiates the Maillard reaction which significantly modifies that composition.
Further Examples of Physical and Chemical Changes
To reinforce the understanding of these concepts, let's examine additional examples.
Physical Changes:
- Dissolving salt in water: The salt disappears into the water, forming a solution, but it retains its chemical identity. The salt can be recovered through evaporation.
- Crushing a can: The shape of the can is altered, but the aluminum remains aluminum.
- Boiling water: Water changes from liquid to gas (steam) but remains water (H₂O).
- Cutting an apple: The apple is physically divided into smaller pieces, but its chemical composition remains the same.
Chemical Changes:
- Burning a candle: The candle wax reacts with oxygen to produce carbon dioxide and water, along with heat and light. This is a combustion reaction.
- Digesting food: Complex food molecules are broken down into simpler molecules through enzymatic reactions.
- Baking bread: Yeast fermentation and the heating process transform flour, water, and other ingredients into a complex mixture of molecules.
- Rusting iron: Iron reacts with oxygen and water to form iron oxide (rust), a different chemical substance.
Conclusion: Melting Butter is Primarily a Physical Change
In summary, the melting of butter is primarily a physical change. The chemical composition of the butter, consisting mainly of triglycerides, remains largely unchanged. Only the physical state transitions from solid to liquid. However, it's vital to acknowledge that prolonged heating at high temperatures can initiate the Maillard reaction, a separate chemical change resulting in browning and the formation of new flavor and aroma compounds. This distinction is crucial for a complete understanding of the processes involved. The initial melting, however, in its pure form, remains firmly in the realm of physical changes. By distinguishing between these processes, we gain a deeper appreciation for the complex interplay of physical and chemical transformations in everyday phenomena.
Latest Posts
Latest Posts
-
Is 20 A Multiple Of 10
Apr 01, 2025
-
What Are Rows In Periodic Table
Apr 01, 2025
-
Sound Waves Cannot Travel In A Vacuum Because
Apr 01, 2025
-
What Is The Difference Between Place And Value
Apr 01, 2025
-
What Is 15 Percent Of 200
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Is Melting Butter Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
