Is Covalent Bond Between Two Nonmetals
Juapaving
Apr 06, 2025 · 5 min read
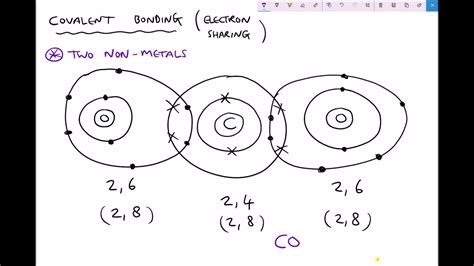
Table of Contents
Is a Covalent Bond Between Two Nonmetals? A Deep Dive into Chemical Bonding
The simple answer is yes, a covalent bond is almost always formed between two nonmetals. Understanding why requires a deeper dive into the nature of atoms, electrons, and the fundamental forces that govern chemical bonding. This comprehensive guide explores the intricacies of covalent bonds, explaining their formation, characteristics, and the exceptions to this rule. We'll also examine the differences between covalent and other types of bonding, providing a solid foundation for understanding chemical interactions.
Understanding Nonmetals and Their Properties
Before delving into covalent bonds, let's establish a clear understanding of nonmetals. These elements are located on the right-hand side of the periodic table, and their properties are largely defined by their electron configuration. Nonmetals generally have high electronegativity, meaning they have a strong tendency to attract electrons. They are typically poor conductors of heat and electricity, often existing as gases, brittle solids, or low-melting-point liquids.
Examples of nonmetals include:
- Oxygen (O): Crucial for respiration and forming many essential compounds.
- Carbon (C): The building block of life, forming the backbone of organic molecules.
- Nitrogen (N): A major component of the atmosphere and essential for protein synthesis.
- Chlorine (Cl): A potent disinfectant and crucial element in many chemical processes.
- Fluorine (F): The most electronegative element, used in various applications including dentistry.
The Mechanics of Covalent Bonding
Covalent bonding arises from the sharing of electrons between two nonmetal atoms. Unlike ionic bonding, where one atom transfers electrons to another, covalent bonding involves a more collaborative arrangement. Both atoms involved contribute valence electrons to form a shared electron pair, creating a stable outer electron shell for both. This shared pair of electrons is attracted to the nuclei of both atoms, holding them together.
The Role of Valence Electrons
Valence electrons are the outermost electrons in an atom. These electrons are crucial in chemical bonding because they are the ones most readily involved in interactions with other atoms. Nonmetals generally have relatively high numbers of valence electrons, typically needing to gain a few electrons to achieve a stable octet (eight electrons in their outermost shell) - a configuration similar to the noble gases. By sharing electrons, nonmetal atoms can achieve this stable octet configuration, thereby lowering their overall energy and increasing their stability.
Single, Double, and Triple Bonds
The number of shared electron pairs between atoms determines the bond order:
- Single Bond: One shared electron pair (e.g., in H₂).
- Double Bond: Two shared electron pairs (e.g., in O₂).
- Triple Bond: Three shared electron pairs (e.g., in N₂).
The greater the number of shared electron pairs, the stronger and shorter the covalent bond.
Visualizing Covalent Bonds: Lewis Dot Structures
Lewis dot structures provide a simple yet powerful way to visualize covalent bonds. These diagrams represent valence electrons as dots surrounding the atomic symbol. Shared electron pairs are represented by lines connecting the atoms. For example, the Lewis dot structure for methane (CH₄) shows carbon sharing four electrons with four hydrogen atoms, resulting in four single covalent bonds.
Polar and Nonpolar Covalent Bonds
The electronegativity difference between the bonded atoms plays a crucial role in determining the nature of the covalent bond:
-
Nonpolar Covalent Bonds: These bonds occur when two atoms of the same element or atoms with very similar electronegativities share electrons equally. The electron density is evenly distributed between the atoms. Examples include H₂, O₂, and Cl₂.
-
Polar Covalent Bonds: These bonds occur when two atoms with significantly different electronegativities share electrons unequally. The more electronegative atom attracts the shared electrons more strongly, resulting in a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
Exceptions to the Rule: Covalent Bonds Involving Metals
While the vast majority of covalent bonds occur between two nonmetals, there are some exceptions. Certain metal atoms, particularly those with relatively high electronegativity, can form covalent bonds with nonmetals. These are often referred to as polar covalent bonds with significant ionic character. The degree of covalent or ionic character depends on the electronegativity difference between the metal and nonmetal. For example, some organometallic compounds exhibit covalent bonding between metals and carbon atoms.
Distinguishing Covalent Bonds from Other Bond Types
It's essential to differentiate covalent bonding from other types of chemical bonding:
-
Ionic Bonding: This occurs when one atom transfers one or more electrons to another atom, forming ions with opposite charges that attract each other electrostatically. This usually happens between a metal and a nonmetal. Sodium chloride (NaCl) is a classic example.
-
Metallic Bonding: This type of bonding occurs in metals, where valence electrons are delocalized and form a "sea" of electrons that are shared among all the metal atoms. This accounts for the high conductivity of metals.
Importance of Covalent Bonding in Chemistry and Biology
Covalent bonds are fundamental to the structure and function of many molecules essential for life and industrial processes. Here are just a few examples:
-
Organic Chemistry: The vast majority of organic compounds, including carbohydrates, lipids, proteins, and nucleic acids, are held together by covalent bonds. The versatility of carbon in forming covalent bonds with itself and other atoms allows for the incredible diversity of organic molecules.
-
Polymer Chemistry: Polymers are large molecules composed of repeating subunits linked together by covalent bonds. Many common materials, like plastics and synthetic fibers, are made of polymers.
-
Biochemistry: Covalent bonds play a critical role in the structure of DNA and proteins, dictating their functions in biological systems.
Conclusion: The Ubiquity of Covalent Bonds Between Nonmetals
In summary, covalent bonds are the predominant type of bond formed between two nonmetals. The sharing of electrons to achieve a stable octet configuration drives the formation of these bonds, leading to a diverse array of molecules with varying properties. While exceptions exist, the rule remains a strong guiding principle in understanding chemical bonding and molecular structure. The implications of covalent bonding span various scientific disciplines, from understanding the intricacies of life to developing advanced materials. Further exploration into this fundamental concept will continue to yield valuable insights into the behavior of matter. Understanding the nuances of covalent bonding is crucial for anyone pursuing studies in chemistry, biology, or materials science.
Latest Posts
Latest Posts
-
How To Calculate Semester Grade Without Final
Apr 08, 2025
-
How To Convert Cubic Feet Into Inches
Apr 08, 2025
-
Is Wavelength And Energy Directly Proportional
Apr 08, 2025
-
2 3 As A Improper Fraction
Apr 08, 2025
-
How Is Plant Cell Mitosis Different From Animal Cell Mitosis
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Is Covalent Bond Between Two Nonmetals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.