Is Charcoal And Coal The Same
Juapaving
Mar 14, 2025 · 5 min read
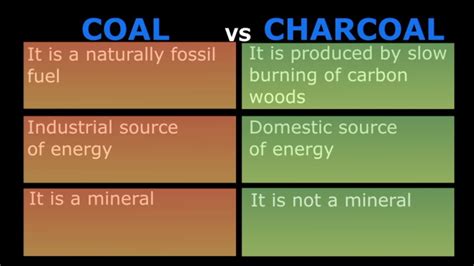
Table of Contents
Is Charcoal and Coal the Same? Unpacking the Differences Between These Two Materials
The terms "charcoal" and "coal" are often used interchangeably, leading to confusion about their distinct properties and origins. While both are carbonaceous materials used for fuel and other applications, understanding their fundamental differences is crucial. This comprehensive guide delves into the intricacies of charcoal and coal, exploring their formation, composition, properties, and applications. We'll uncover why they are definitively not the same, clarifying any misconceptions along the way.
Understanding Charcoal: A Product of Incomplete Combustion
Charcoal is a lightweight black carbon residue produced by strongly heating wood or other plant-based materials in the absence of oxygen. This process, known as pyrolysis or carbonization, drives off volatile compounds like water, hydrogen, and methane, leaving behind a porous carbon structure. The resulting charcoal retains the original material's structure but is significantly denser and more carbon-rich.
The Pyrolysis Process: A Closer Look
The creation of charcoal is a carefully controlled process. Different techniques yield charcoal with varying properties. Traditional methods involve building mounds of wood and covering them with earth, allowing the wood to smolder slowly. More modern techniques utilize kilns or retorts to precisely manage temperature and airflow, optimizing the process for specific charcoal qualities. The absence of oxygen is paramount; if oxygen were abundant, the wood would simply burn, producing ash and gases, instead of charcoal.
Key Characteristics of Charcoal:
- Porous Structure: Charcoal's porous nature is a key characteristic, contributing to its high surface area. This high surface area is crucial for its absorbent properties, making it useful in filtration and other applications.
- High Carbon Content: Charcoal is primarily composed of carbon, typically around 80-90%. The remaining portion consists of small amounts of other elements like hydrogen, oxygen, and nitrogen.
- Lightweight: Compared to coal, charcoal is significantly lighter, making it easier to handle and transport.
- Versatile Applications: Charcoal is used in a wide array of applications, including grilling, drawing, water filtration, and industrial processes.
Types of Charcoal:
The type of wood used and the pyrolysis method employed significantly affect the resulting charcoal's properties. For example, hardwood charcoal generally burns longer and hotter than softwood charcoal, making it more desirable for grilling. Activated charcoal, a highly processed form of charcoal, boasts an exceptionally large surface area, making it ideal for applications requiring strong adsorption, such as water purification.
Exploring Coal: A Fossil Fuel Formed Over Millions of Years
Coal, unlike charcoal, is a sedimentary rock formed from the remains of ancient plant matter subjected to intense pressure and heat over millions of years. This process of fossilization transformed the organic matter into a carbon-rich rock. Unlike charcoal, coal is a fossil fuel, meaning it’s a non-renewable resource formed over geological timescales.
Coal Formation: A Geological Perspective
The formation of coal is a complex geological process involving several stages:
- Accumulation: Large quantities of plant matter, primarily from swamps and bogs, accumulate in waterlogged environments, preventing decomposition.
- Peat Formation: Under anaerobic conditions (lack of oxygen), the plant matter partially decomposes, forming peat, a spongy material rich in organic matter.
- Compaction and Diagenesis: Over time, layers of sediment bury the peat, subjecting it to increasing pressure and temperature. This process, known as diagenesis, transforms the peat into lignite, the lowest rank of coal.
- Coalification: Continued pressure and heat further alter the lignite, increasing its carbon content and producing higher-rank coals: sub-bituminous, bituminous, and anthracite. Anthracite, the highest rank of coal, has the highest carbon content and energy density.
Key Characteristics of Coal:
- High Carbon Content (Variable): The carbon content in coal varies depending on its rank, ranging from approximately 60% in lignite to over 90% in anthracite.
- High Energy Density: Coal is a high-energy fuel, releasing significant heat upon combustion.
- Different Ranks and Properties: The different ranks of coal (lignite, sub-bituminous, bituminous, anthracite) possess varying properties, affecting their suitability for different applications. For example, anthracite, with its high carbon content, burns cleaner and hotter than lignite.
- Non-Renewable Resource: Coal is a finite resource; once it’s consumed, it cannot be replenished within human timescales.
- Environmental Concerns: Coal combustion releases significant amounts of greenhouse gases (carbon dioxide, methane) contributing to climate change, and pollutants like sulfur dioxide and nitrogen oxides, leading to air pollution and acid rain.
Key Differences Between Charcoal and Coal: A Side-by-Side Comparison
| Feature | Charcoal | Coal |
|---|---|---|
| Origin | Pyrolysis of plant matter | Fossilized plant matter |
| Formation Time | Hours to days | Millions of years |
| Carbon Content | 80-90% | 60-90% (varies with rank) |
| Renewable | Potentially renewable (from sustainable wood sources) | Non-renewable |
| Energy Density | Lower than coal | Higher than charcoal |
| Appearance | Black, porous | Black to dark brown, often layered |
| Combustion | Burns relatively quickly and cleanly (depending on the quality) | Burns less cleanly, releasing more pollutants |
| Applications | Grilling, drawing, filtration, industrial processes | Electricity generation, steel production, fuel |
| Environmental Impact | Lower than coal, but still produces some emissions | High environmental impact due to greenhouse gas and pollutant emissions |
Debunking Common Myths: Are Charcoal and Coal Interchangeable?
No, charcoal and coal are distinctly different materials, despite their shared characteristic of being primarily carbon-based. Their formation processes, properties, and applications are vastly different. Using them interchangeably is inaccurate and ignores crucial distinctions that significantly impact their uses and environmental implications.
Addressing the Confusion: Why the Misconception Exists
The confusion often stems from their shared use as fuels. Both can be burned to produce heat, and both are black, carbonaceous materials. However, this superficial similarity masks fundamental differences in their composition, formation, and environmental impact.
Conclusion: Understanding the Nuances
The differences between charcoal and coal are significant, extending beyond their superficial similarities. Charcoal is a product of controlled pyrolysis, a relatively quick process producing a porous, lightweight material with diverse applications. Coal, on the other hand, is a fossil fuel formed over geological timescales, possessing different ranks with varying properties and significant environmental implications. Recognizing these differences is crucial for informed decision-making concerning their uses and environmental consequences. Choosing sustainable and environmentally responsible options whenever possible is essential for mitigating the negative impacts associated with carbon-based fuels. By understanding the distinctions, we can make more informed choices and appreciate the unique properties and applications of both charcoal and coal.
Latest Posts
Latest Posts
-
What Is Pure Breeding In Genetics
May 09, 2025
-
Is 6 A Multiple Of 12
May 09, 2025
-
Molal Boiling Point Elevation Constant Table
May 09, 2025
-
What Is The Improper Fraction For 2 3 4
May 09, 2025
-
120 In Equals How Many Feet
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Charcoal And Coal The Same . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.