Is Boiling Water Endothermic Or Exothermic
Juapaving
Mar 04, 2025 · 5 min read
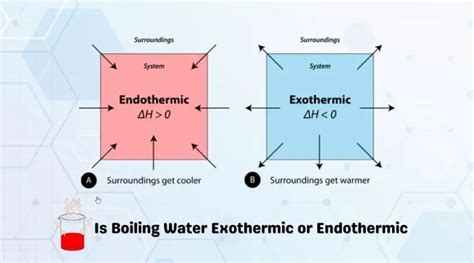
Table of Contents
Is Boiling Water Endothermic or Exothermic? Understanding Heat Transfer in Phase Changes
The question of whether boiling water is an endothermic or exothermic process is a fundamental concept in chemistry and thermodynamics. Understanding this seemingly simple process reveals deeper insights into heat transfer, energy changes, and the behavior of matter at a molecular level. This comprehensive guide will delve into the intricacies of boiling water, explaining why it's classified as endothermic and exploring the related scientific principles.
Understanding Endothermic and Exothermic Reactions
Before we dive into the specifics of boiling water, let's clarify the definitions of endothermic and exothermic processes. These terms describe the direction of heat flow during a reaction or phase change:
-
Exothermic Reactions: In exothermic processes, heat is released to the surroundings. The system's energy decreases, and the surroundings become warmer. Think of combustion (burning) – it releases heat and light.
-
Endothermic Reactions: In endothermic processes, heat is absorbed from the surroundings. The system's energy increases, and the surroundings become cooler. Melting ice is a classic example; it absorbs heat from its environment to change from a solid to a liquid.
The Boiling Process: A Detailed Look
Boiling is a phase transition where a liquid turns into a gas (vaporization). Water, specifically, transitions from liquid water (H₂O) to water vapor (steam). This process requires a significant input of energy to overcome the intermolecular forces holding the water molecules together in the liquid state. These forces, primarily hydrogen bonds, need to be broken for the molecules to escape into the gaseous phase.
The Role of Heat Energy
The heat energy supplied during boiling is not simply increasing the temperature of the water. While the water does initially increase in temperature, once it reaches its boiling point (100°C or 212°F at standard atmospheric pressure), adding more heat doesn't raise the temperature further. Instead, that additional energy is used exclusively to break the hydrogen bonds and convert liquid water into gaseous water vapor.
Molecular Perspective
Imagine the water molecules as closely packed individuals in a crowded room (liquid state). They are constantly moving and colliding, but their movement is restricted by their proximity. Adding heat provides the energy for these molecules to overcome their attraction to each other and escape the "room" (transition to the gaseous state). They gain enough kinetic energy to break free from the liquid phase and become independent gas molecules moving more freely.
Why Boiling Water is Endothermic
Because boiling water requires a constant input of heat energy to sustain the phase transition from liquid to gas, it is unequivocally an endothermic process. The system (the water) is absorbing energy from its surroundings (the heat source, like a stove or burner). If the heat source were removed, the boiling would cease.
Evidence for Endothermicity
Several observations support the endothermic nature of boiling:
-
Temperature Plateau: As mentioned earlier, the temperature of boiling water remains constant at its boiling point, even with continued heat input. This is strong evidence that the added energy is being used for a phase change, not a temperature increase.
-
Cooling Effect: If you've ever boiled water on a stovetop, you might have noticed that the area surrounding the pot can feel cooler. This is because the water is absorbing heat from its surroundings, effectively cooling the nearby environment. This cooling effect is a hallmark of endothermic processes.
-
Heat Capacity and Latent Heat: The amount of heat required to boil a certain mass of water is quantified by its latent heat of vaporization. This is the energy needed to change the phase of a substance without a temperature change. The high latent heat of vaporization for water underscores the significant energy input required for boiling, confirming its endothermic nature.
Factors Affecting Boiling Point
While the boiling point of water is typically 100°C at standard atmospheric pressure, several factors can influence it:
-
Pressure: Lower atmospheric pressure (like at high altitudes) lowers the boiling point of water. Conversely, higher pressure increases the boiling point. This is because lower pressure reduces the force holding the water molecules together, requiring less energy to transition to the gaseous phase.
-
Dissolved Impurities: The presence of dissolved substances (salts, sugars, etc.) in the water can slightly elevate its boiling point. This is known as boiling point elevation. The dissolved particles interfere with the water molecules' ability to escape into the gaseous phase, necessitating slightly higher energy input.
-
Presence of Bubbles: The formation and release of bubbles during boiling are crucial aspects of the process. Bubbles act as nucleation sites, providing surfaces for vaporization to occur. The presence of impurities or surface imperfections can affect bubble formation and, thus, the boiling process itself.
Applications and Significance
Understanding the endothermic nature of boiling water has far-reaching applications in various fields:
-
Cooking: Boiling is essential in various cooking methods, from boiling pasta to steaming vegetables. The heat transfer and phase change involved directly impact the cooking process.
-
Industrial Processes: Boiling is used in many industrial processes for purposes such as sterilization, purification, and separation of substances. The energy requirements and efficiency of boiling are critical considerations.
-
Meteorology: The boiling and evaporation of water play critical roles in weather patterns, influencing cloud formation, precipitation, and global climate.
-
Power Generation: Boiling water is fundamental to many power generation systems, particularly in steam-powered plants. The energy conversion process relies heavily on the endothermic nature of boiling.
Conclusion: A Fundamental Process with Broader Implications
The seemingly simple act of boiling water reveals profound principles in thermodynamics and phase transitions. Its classification as an endothermic process is firmly established through experimental evidence and a molecular-level understanding of the energy changes involved. This seemingly simple concept has broad-reaching applications across various scientific and industrial domains, highlighting the importance of grasping the fundamentals of heat transfer and energy changes in the natural world and our technological advancements. Understanding the endothermic nature of boiling water helps us to appreciate the intricate interplay of energy and matter, underpinning many essential processes we encounter daily. From cooking a meal to generating electricity, the fundamental principles discussed here are at play, underscoring the importance of comprehending these basic, yet profound, aspects of physics and chemistry. Continued investigation into boiling and phase changes promises to yield even further insights into the complex behaviors of materials and their interaction with energy.
Latest Posts
Latest Posts
-
How Many Centimeters Is 6 Ft
Mar 04, 2025
-
Which Of The Following Statements Is Not True
Mar 04, 2025
-
What Is 2 3 As A Percent
Mar 04, 2025
-
How Many Feet Is 36 Inches
Mar 04, 2025
-
What Is The Major Product Of The Following Reaction
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water Endothermic Or Exothermic . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
