Is Boiling Water A Physical Or Chemical Change
Juapaving
Apr 06, 2025 · 5 min read
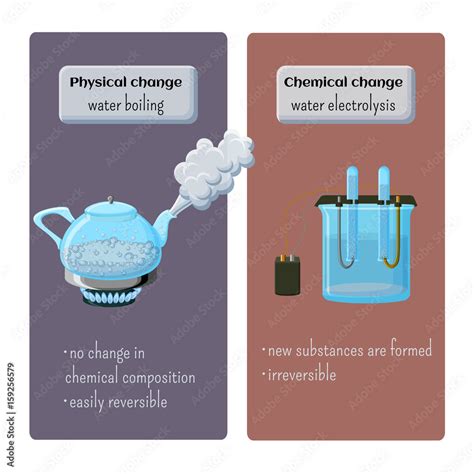
Table of Contents
Is Boiling Water a Physical or Chemical Change? A Comprehensive Look
The question of whether boiling water represents a physical or chemical change is a common one, often sparking debate among students and science enthusiasts alike. The answer, however, is more nuanced than a simple "yes" or "no." Understanding the difference between physical and chemical changes is crucial to grasping this seemingly straightforward phenomenon. This article will delve deep into the process of boiling water, examining the evidence and arguments from both sides to definitively answer the question and illuminate the underlying principles.
Defining Physical and Chemical Changes
Before tackling the boiling water conundrum, let's establish clear definitions for physical and chemical changes:
Physical Change: A physical change alters the form or appearance of a substance but does not change its chemical composition. The substance remains the same; only its physical properties (like shape, size, or state) are modified. Examples include melting ice, tearing paper, or dissolving sugar in water. Crucially, these changes are often reversible.
Chemical Change: A chemical change, also known as a chemical reaction, involves a transformation in the chemical composition of a substance. New substances with different properties are formed, and this process is often irreversible. Examples include burning wood, rusting iron, or baking a cake. These changes typically involve the breaking and forming of chemical bonds.
Analyzing the Boiling of Water
Now, let's analyze the process of boiling water through the lens of these definitions. When water boils, it transitions from its liquid state to its gaseous state (water vapor or steam). This transition involves a significant change in its physical properties:
- State of matter: The most obvious change is the alteration of the state from liquid to gas.
- Density: Gaseous water is significantly less dense than liquid water.
- Volume: Water expands considerably when it changes to steam.
- Temperature: Boiling point is reached at a specific temperature (100°C or 212°F at standard atmospheric pressure).
However, the chemical composition of the water remains unchanged. The water molecules (H₂O) are still composed of two hydrogen atoms and one oxygen atom, bonded together covalently. No new substances are formed during boiling. The steam produced is simply water in a gaseous phase. You can condense the steam back into liquid water, demonstrating the reversibility of the process.
Arguments for Boiling Water as a Physical Change
The evidence strongly suggests that boiling water is a physical change. The key arguments supporting this classification are:
- No new substance is formed: The chemical formula remains H₂O throughout the entire process.
- Reversibility: The process can be reversed by cooling the steam, condensing it back into liquid water. This reversibility is a hallmark of physical changes.
- Only physical properties are altered: Changes occur in the state, density, volume, and temperature, all of which are physical properties. The inherent chemical nature of the water molecule remains intact.
Addressing Potential Counterarguments
Some might argue that the breaking of hydrogen bonds between water molecules during boiling constitutes a chemical change. However, it's crucial to understand that hydrogen bonds are intermolecular forces—forces between molecules, not intramolecular forces—forces within a molecule. Breaking intermolecular forces does not alter the chemical composition of the molecules themselves. These bonds reform when the steam condenses, further highlighting the reversibility.
Another point of contention might be the energy input required to boil water. This energy breaks the hydrogen bonds, enabling the phase transition. However, energy input itself does not automatically signify a chemical change. Many physical changes require energy input (like melting ice), but they don't alter the chemical makeup of the substance.
Boiling Water: A Physical Change in the Context of Other Processes
To further solidify the argument, let's compare boiling water to other processes:
- Electrolysis of water: This process uses electricity to decompose water into hydrogen and oxygen gases (2H₂O → 2H₂ + O₂). This is a chemical change because new substances are formed.
- Burning hydrogen: When hydrogen gas reacts with oxygen, it forms water (2H₂ + O₂ → 2H₂O). This is a chemical change due to the formation of a new substance.
These examples illustrate the clear distinction between physical and chemical changes. Boiling water differs significantly from these examples; it doesn't involve the formation of new substances or the breaking of intramolecular bonds.
The Importance of Understanding Phase Transitions
Boiling water exemplifies a phase transition, a physical process where a substance changes from one state of matter to another. Understanding phase transitions is fundamental in various scientific fields, including:
- Meteorology: Phase transitions of water are crucial for understanding weather patterns, cloud formation, and precipitation.
- Chemistry: Phase transitions are essential in various chemical processes and separations.
- Engineering: Phase transitions are important in designing and optimizing various systems, including power generation and refrigeration.
Conclusion: Boiling Water is a Physical Change
Based on the evidence and arguments presented, it's clear that boiling water is primarily a physical change. While energy input and the breaking of intermolecular forces are involved, the chemical composition of the water remains unchanged. The process is reversible, and only the physical properties of the water are altered. Understanding this distinction helps solidify our grasp of fundamental scientific principles and their applications in various fields.
This definitive answer is crucial for students and anyone interested in the fundamentals of chemistry and physics. By understanding the nuances of physical and chemical changes, a broader understanding of the world around us is achieved, helping us to appreciate the intricacies of everyday phenomena. The boiling of water, while seemingly simple, provides a valuable lens through which to explore these fundamental concepts.
Latest Posts
Latest Posts
-
The Functional Unit Of Kidney Is
Apr 08, 2025
-
What Is The Multiples Of 50
Apr 08, 2025
-
Sin 18 Degrees Sin 54 Degrees
Apr 08, 2025
-
The First Five Multiples Of 9
Apr 08, 2025
-
Is A Mouse Software Or Hardware
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Is Boiling Water A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.