Is Blood A Pure Substance Or A Mixture
Juapaving
Mar 21, 2025 · 5 min read
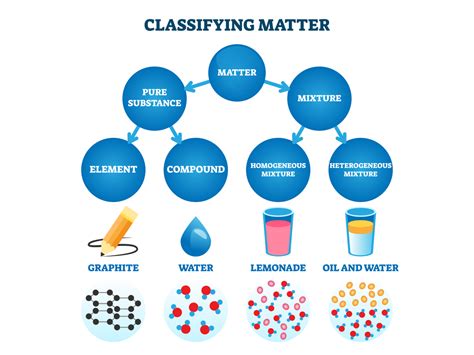
Table of Contents
Is Blood a Pure Substance or a Mixture? A Comprehensive Look
Blood, the vibrant red fluid coursing through our veins and arteries, is far more complex than it initially appears. This vital substance, responsible for transporting oxygen, nutrients, and hormones throughout the body, while simultaneously removing waste products, is a captivating subject for scientific inquiry. One fundamental question that often arises is: is blood a pure substance or a mixture? The answer, as we'll explore in detail, is unequivocally a mixture. Understanding why requires a deeper dive into the composition and properties of blood.
Understanding Pure Substances and Mixtures
Before classifying blood, let's establish a clear understanding of the definitions of pure substances and mixtures.
Pure Substances: The Building Blocks
A pure substance is a form of matter that has a constant composition and properties throughout the sample. It cannot be separated into other kinds of matter by any physical process. Pure substances are further categorized into elements and compounds.
-
Elements: Elements are pure substances that consist of only one type of atom. Examples include oxygen (O), iron (Fe), and gold (Au). They cannot be broken down into simpler substances by chemical means.
-
Compounds: Compounds are pure substances composed of two or more elements chemically bonded in fixed proportions. Water (H₂O), table salt (NaCl), and carbon dioxide (CO₂) are examples of compounds. These can only be separated into their constituent elements through chemical reactions.
Mixtures: A Blend of Components
A mixture, unlike a pure substance, is composed of two or more substances physically combined. These substances retain their individual chemical identities and can be separated by physical methods like filtration, distillation, or evaporation. Mixtures are further categorized into homogeneous and heterogeneous mixtures.
-
Homogeneous Mixtures: In a homogeneous mixture, the components are uniformly distributed throughout the sample. The composition is consistent throughout. Examples include saltwater, air, and sugar dissolved in water.
-
Heterogeneous Mixtures: In a heterogeneous mixture, the components are not uniformly distributed. Different regions of the sample have different compositions. Examples include sand and water, oil and water, and a salad.
The Composition of Blood: A Complex Mixture
Blood, with its myriad components, clearly falls under the category of a mixture. It's a complex homogeneous mixture (appearing uniform to the naked eye), though closer examination reveals a suspension of various cells and particles within a liquid medium. Let's delve into the key components:
1. Plasma: The Liquid Matrix
Plasma constitutes approximately 55% of blood volume and serves as the liquid medium in which the other blood components are suspended. It's primarily composed of water (about 92%), but it's far from just water. Plasma is a complex solution containing:
-
Proteins: A diverse array of proteins, including albumin, globulins, and fibrinogen, perform crucial roles in maintaining osmotic pressure, transporting substances, and blood clotting.
-
Electrolytes: Ions such as sodium (Na⁺), potassium (K⁺), chloride (Cl⁻), calcium (Ca²⁺), and bicarbonate (HCO₃⁻) are vital for maintaining proper fluid balance, nerve impulse transmission, and muscle contraction.
-
Nutrients: Glucose, amino acids, lipids, and vitamins are transported by plasma to various tissues throughout the body.
-
Waste Products: Urea, creatinine, and uric acid, metabolic byproducts, are carried by plasma to the kidneys for excretion.
-
Hormones: Chemical messengers like insulin and adrenaline are transported via plasma to their target cells.
-
Gases: Dissolved oxygen (O₂) and carbon dioxide (CO₂) are carried by plasma, although hemoglobin in red blood cells is the primary oxygen transporter.
2. Formed Elements: The Cellular Components
The remaining 45% of blood volume consists of the "formed elements," which are the cellular components of blood. These include:
-
Red Blood Cells (Erythrocytes): These are the most abundant cells in blood, responsible for oxygen transport. They contain hemoglobin, an iron-containing protein that binds to oxygen in the lungs and releases it to tissues.
-
White Blood Cells (Leukocytes): These are part of the body's immune system, defending against infection and disease. There are several types of leukocytes, each with specific roles in immune response.
-
Platelets (Thrombocytes): These small cell fragments play a crucial role in blood clotting, preventing excessive bleeding.
Separating Blood Components: Evidence of a Mixture
The fact that blood's components can be separated using physical methods further solidifies its classification as a mixture. Techniques like centrifugation are commonly used in laboratories to separate blood into its different components:
- Centrifugation: Spinning blood at high speeds in a centrifuge separates the denser formed elements (red blood cells, white blood cells, and platelets) from the less dense plasma. This process doesn't involve any chemical reactions; it relies solely on differences in density.
Why the "Mixture" Classification is Crucial
Understanding blood as a mixture is paramount for several reasons:
-
Medical Diagnosis: Analyzing the different components of blood, such as blood cell counts, plasma protein levels, and electrolyte concentrations, is crucial for diagnosing various medical conditions.
-
Blood Transfusions: The compatibility of different blood types is determined by the presence or absence of specific antigens on the surface of red blood cells. Understanding blood as a mixture is essential for safe blood transfusions.
-
Research and Development: The study of individual components of blood has led to significant advancements in the treatment of various diseases, including blood disorders and immune deficiencies.
Conclusion: Blood – A Dynamic and Essential Mixture
In conclusion, blood is undeniably a mixture, a complex and dynamic blend of various substances. Its classification as a homogeneous mixture, with its intricate array of liquid and cellular components, highlights its sophisticated role in maintaining life. The ability to separate these components through physical means underscores the distinct nature of its constituents and reinforces the importance of recognizing blood as a mixture for scientific understanding and medical applications. Further investigation into the specific properties and functions of each component continues to reveal the awe-inspiring complexity of this vital bodily fluid. The ongoing research expands our knowledge and fuels advancements in healthcare, further emphasizing the critical understanding of blood as a complex and fascinating mixture.
Latest Posts
Latest Posts
-
Which Of The Following Is Strong Electrolyte
May 09, 2025
-
Why Do Skeletal Muscles Work In Pairs
May 09, 2025
-
Compare And Contrast Elisa And Western Blotting
May 09, 2025
-
Double Layered Membrane On The Outside Of The Heart
May 09, 2025
-
Is Atp Hydrolysis Endergonic Or Exergonic
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Blood A Pure Substance Or A Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.