Is Alcohol A Mixture Or Substance
Juapaving
Mar 18, 2025 · 5 min read
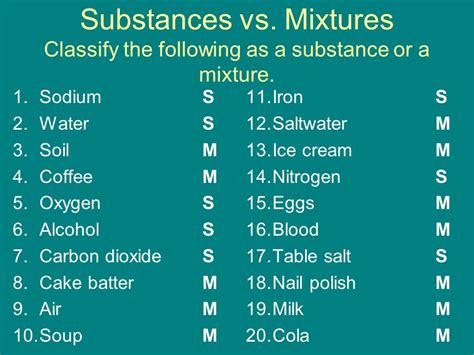
Table of Contents
Is Alcohol a Mixture or a Substance? A Deep Dive into the Chemistry of Alcoholic Beverages
The question, "Is alcohol a mixture or a substance?" seems deceptively simple. However, the answer depends on how you define "alcohol" and the context in which you're asking. Let's delve into the fascinating chemistry of alcoholic beverages to unravel this seemingly straightforward query.
Understanding the Terms: Mixture vs. Substance
Before we can classify alcohol, we need to understand the fundamental differences between a mixture and a substance.
Substance: A substance is a form of matter that has a uniform and definite composition. It's chemically pure, meaning it's made up of only one type of atom or molecule. Examples include pure water (H₂O), pure gold (Au), and pure table salt (NaCl). Substances have distinct physical and chemical properties.
Mixture: A mixture is a combination of two or more substances that are physically combined but not chemically bonded. The components of a mixture retain their individual properties, and the composition of a mixture can vary. Examples include air (a mixture of gases), saltwater (a mixture of salt and water), and a salad (a mixture of vegetables). Mixtures can be homogeneous (uniform throughout, like saltwater) or heterogeneous (non-uniform, like a salad).
The Case of Ethanol: The "Alcohol" in Alcoholic Beverages
When most people refer to "alcohol," they're talking about ethanol (C₂H₅OH), the type of alcohol found in alcoholic beverages. Ethanol is a pure substance. It's a specific molecule with a consistent chemical formula and set of properties. It's not a combination of different substances; it's a single chemical compound.
Properties of Ethanol: A Pure Substance at its Core
Ethanol's distinct properties are what make it suitable for consumption (in moderation) and use in various industrial applications. These properties include:
- Colorless liquid: Pure ethanol is a clear, colorless liquid.
- Flammable: Ethanol readily burns in the presence of oxygen.
- Characteristic odor: It has a distinct, slightly sweet smell.
- Solvency: Ethanol is a good solvent, meaning it can dissolve many substances.
- Toxicity: While it can be consumed in moderation, excessive ethanol consumption is toxic and can lead to health problems.
These consistent and predictable properties solidify ethanol's status as a pure substance.
Alcoholic Beverages: Mixtures, Not Pure Substances
While ethanol itself is a substance, alcoholic beverages are definitively mixtures. They contain ethanol as a primary component, but they also include many other substances, including:
- Water: The water content in alcoholic beverages significantly influences their flavor, body, and alcohol percentage.
- Congeners: These are other alcohols and organic compounds produced during fermentation. They contribute to the flavor, aroma, and color of the beverage. Different types of alcoholic beverages have varying congener profiles, which leads to the diverse taste experiences associated with different drinks such as whiskey, wine, or beer. The presence of congeners is a key distinguishing factor between various types of alcoholic beverages.
- Sugars: Residual sugars from the fermentation process are often present, contributing to sweetness and body. The amount of residual sugar varies widely depending on the type of alcoholic beverage.
- Acids: Various organic acids are present, influencing the taste and overall balance of the beverage. These acids play a critical role in the overall flavor profile.
- Other flavor compounds: Depending on the type of alcoholic beverage, other compounds contribute to the distinctive flavor profile. This can include esters, aldehydes, ketones, and various other organic molecules. These compounds add to the complexity of flavor.
These additional components significantly alter the overall properties of the beverage compared to pure ethanol. The presence of these compounds proves that alcoholic beverages are mixtures, not pure substances.
Examples of Alcoholic Beverages as Mixtures:
- Wine: Wine is a mixture of ethanol, water, various acids (like tartaric acid and malic acid), sugars, and various other flavor compounds derived from grapes and the fermentation process. The specific composition varies based on the grape variety, fermentation techniques, and aging process.
- Beer: Beer is a mixture of ethanol, water, carbon dioxide, hops, barley, and other additives contributing to its flavor and aroma. The process of brewing results in a complex mixture of organic compounds.
- Whiskey: Whiskey is a mixture of ethanol, water, and congeners created during fermentation and aging in oak barrels. The type of grain used, the aging process, and the barrel type all contribute to the diverse range of whiskeys on the market. Each whiskey possesses a unique composition.
- Spirits: Distilled spirits like vodka, gin, and rum are essentially concentrated solutions of ethanol and water, with additional flavor compounds added for specific types of spirits. The distillation process increases the ethanol concentration.
These examples highlight the complexity of alcoholic beverages as mixtures. They are not simply pure ethanol; they are carefully crafted blends of multiple substances.
The Importance of Understanding the Distinction
The distinction between ethanol as a pure substance and alcoholic beverages as mixtures is crucial for several reasons:
- Regulation and Taxation: Governments regulate the alcohol content of beverages, and taxes are often levied based on the ethanol concentration. Understanding that the beverage is a mixture containing varying concentrations of ethanol is necessary for accurate regulations.
- Health Considerations: The effects of consuming alcoholic beverages depend not only on the ethanol content but also on the other components present in the mixture. Congeners, for example, can contribute to hangovers. This underscores the importance of understanding both the pure substance (ethanol) and the mixture (alcoholic beverage).
- Food Science and Technology: Understanding the chemical composition of alcoholic beverages is essential for food scientists and technologists involved in their production and quality control. Knowing the components of the mixture is vital in maintaining consistent quality and ensuring the safety of the beverage.
- Scientific Research: The study of alcoholic beverages requires a thorough understanding of both the pure substance (ethanol) and the complex mixture comprising the beverage. This knowledge is crucial in research related to fermentation processes and health effects.
Conclusion: A nuanced answer
To reiterate, ethanol, the alcohol in alcoholic drinks, is a pure substance. However, alcoholic beverages themselves are mixtures containing ethanol as the primary component along with water, congeners, sugars, acids, and numerous other compounds that contribute to their unique flavor profiles and overall characteristics. The simple question therefore has a nuanced answer; while the fundamental chemical component is a pure substance, the product we ultimately consume is a complex mixture. Understanding this distinction is important for various aspects of the beverage industry, public health, and scientific research.
Latest Posts
Latest Posts
-
Torque And Moment Of Inertia Relationship
Mar 18, 2025
-
Is 1 3 Bigger Than 2 5
Mar 18, 2025
-
Tap Water Is Pure Substance Or Mixture
Mar 18, 2025
-
3 M Is How Many Cm
Mar 18, 2025
-
Two Angles Form A Linear Pair
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Alcohol A Mixture Or Substance . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
