Ionic Compounds Are Composed Of What
Juapaving
Apr 08, 2025 · 6 min read
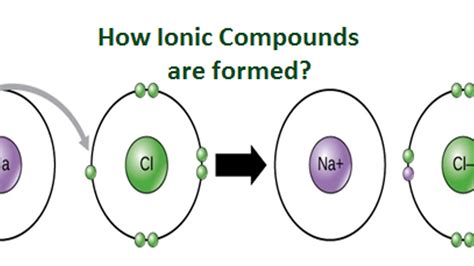
Table of Contents
Ionic Compounds: A Deep Dive into Their Composition and Properties
Ionic compounds are fundamental building blocks of chemistry, found everywhere from the salt shaker on your kitchen table to the intricate mechanisms within your body. Understanding what constitutes these compounds is key to grasping a wide range of chemical and biological processes. This article will delve into the very essence of ionic compounds, exploring their composition, formation, properties, and significance.
What are Ionic Compounds Composed Of?
At their core, ionic compounds are composed of ions. These ions are charged atoms or molecules, formed through the transfer of electrons between atoms. This transfer results in the formation of positively charged ions (cations) and negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions is the driving force behind the formation of the ionic compound and holds the structure together.
Cations: Positively Charged Ions
Cations are formed when an atom loses one or more electrons. This typically occurs with metals, which have a relatively low electronegativity (a measure of an atom's tendency to attract electrons). Metals readily relinquish their valence electrons (the outermost electrons) to achieve a more stable electron configuration, often resembling a noble gas.
For example:
- Sodium (Na), an alkali metal, loses one electron to form a sodium ion (Na⁺).
- Magnesium (Mg), an alkaline earth metal, loses two electrons to form a magnesium ion (Mg²⁺).
- Aluminum (Al) loses three electrons to form an aluminum ion (Al³⁺).
The charge of the cation directly corresponds to the number of electrons lost.
Anions: Negatively Charged Ions
Anions, conversely, are formed when an atom gains one or more electrons. This process commonly occurs with nonmetals, which have higher electronegativities and a stronger attraction for electrons. By gaining electrons, nonmetals achieve a stable electron configuration, often mimicking a noble gas.
Examples include:
- Chlorine (Cl), a halogen, gains one electron to form a chloride ion (Cl⁻).
- Oxygen (O) gains two electrons to form an oxide ion (O²⁻).
- Nitrogen (N) gains three electrons to form a nitride ion (N³⁻).
The negative charge of the anion is equal to the number of electrons gained.
The Formation of Ionic Compounds: A Balancing Act
The formation of an ionic compound is a crucial process driven by the fundamental principle of achieving electrostatic stability. The transfer of electrons between a metal and a nonmetal results in ions with complete outermost electron shells, significantly lowering the overall energy of the system.
Consider the formation of sodium chloride (NaCl), common table salt:
- A sodium atom (Na) loses one electron to become a sodium ion (Na⁺).
- A chlorine atom (Cl) gains one electron to become a chloride ion (Cl⁻).
- The electrostatic attraction between the positively charged Na⁺ and the negatively charged Cl⁻ ions leads to the formation of an ionic bond, creating a crystal lattice structure of sodium chloride.
This electrostatic attraction is incredibly strong, leading to the characteristic properties of ionic compounds, which we will explore in the next section. The ratio of cations to anions in an ionic compound is always such that the overall charge of the compound is neutral. For example, in NaCl, the 1:1 ratio of Na⁺ and Cl⁻ ensures electrical neutrality. In magnesium oxide (MgO), the ratio is 1:1 as well (Mg²⁺ and O²⁻), while in aluminum oxide (Al₂O₃), the ratio is 2:3 to balance the charges.
Beyond Simple Binary Compounds: Polyatomic Ions
The composition of ionic compounds is not limited to simple combinations of one metal cation and one nonmetal anion. Many ionic compounds involve polyatomic ions, which are groups of atoms covalently bonded together that carry an overall charge.
Examples include:
- Nitrate ion (NO₃⁻)
- Sulfate ion (SO₄²⁻)
- Phosphate ion (PO₄³⁻)
- Ammonium ion (NH₄⁺)
These polyatomic ions act as single units within the ionic compound, participating in electrostatic interactions with other ions to maintain overall electrical neutrality. For instance, ammonium nitrate (NH₄NO₃) contains both ammonium (NH₄⁺) and nitrate (NO₃⁻) ions.
Properties of Ionic Compounds: A Consequence of Structure
The strong electrostatic forces between ions in an ionic lattice dictate the characteristic properties of ionic compounds. These properties include:
High Melting and Boiling Points:
The strong ionic bonds require significant energy to overcome, resulting in high melting and boiling points. This is in contrast to covalent compounds, which often have much lower melting and boiling points.
Crystalline Structure:
Ionic compounds typically form regular, repeating crystal lattices. The arrangement of ions in these lattices is determined by the sizes and charges of the ions involved, leading to various crystal structures such as cubic, tetragonal, or hexagonal.
Brittle Nature:
The rigid crystal structure of ionic compounds makes them brittle. When subjected to stress, the ions can shift, leading to repulsion between ions of the same charge, causing the crystal to fracture.
Electrical Conductivity:
Ionic compounds are generally good conductors of electricity when molten (liquid) or dissolved in water (aqueous solution). In these states, the ions are free to move and carry an electric current. However, in their solid crystalline state, the ions are fixed in position and cannot conduct electricity.
Solubility:
The solubility of ionic compounds varies greatly depending on the nature of the ions involved and the solvent. Many ionic compounds are soluble in polar solvents like water, where the polar water molecules can interact with and surround the ions, disrupting the ionic lattice.
Significance of Ionic Compounds: Their Roles in Life and Industry
Ionic compounds play vital roles in numerous aspects of life and industry:
Biological Systems:
Many essential biological processes rely on ionic compounds. For example, sodium and potassium ions are crucial for nerve impulse transmission, while calcium ions are essential for muscle contraction and bone formation. Electrolyte balance, involving various ionic compounds, is critical for proper bodily function.
Industrial Applications:
Ionic compounds have widespread industrial applications. Sodium chloride (NaCl) is used in food preservation, water treatment, and various chemical processes. Other ionic compounds find applications in fertilizers, construction materials, pharmaceuticals, and many other industries.
Conclusion: A Deeper Understanding of Ionic Compounds
This comprehensive exploration has illuminated the fundamental composition of ionic compounds, their formation mechanism, and their key properties. The transfer of electrons between metals and nonmetals results in the formation of oppositely charged ions held together by strong electrostatic forces. These strong bonds give rise to the characteristic high melting points, brittle nature, and electrical conductivity (in molten or aqueous states) of ionic compounds. Their widespread significance in biological systems and numerous industrial applications underscores their crucial role in our world. Understanding the composition and behavior of ionic compounds is fundamental to comprehending many aspects of chemistry and its applications in diverse fields.
Latest Posts
Latest Posts
-
Two Angles That Have The Same Measure
Apr 08, 2025
-
Adjectives That Start With The Letter D
Apr 08, 2025
-
Functional Group With A Face Funny
Apr 08, 2025
-
What Do You Call A Group Of Lions
Apr 08, 2025
-
Whats The Lcm Of 9 And 12
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Ionic Compounds Are Composed Of What . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.