If You Have 155 Ml Solution Of A 0.762
Juapaving
May 30, 2025 · 5 min read
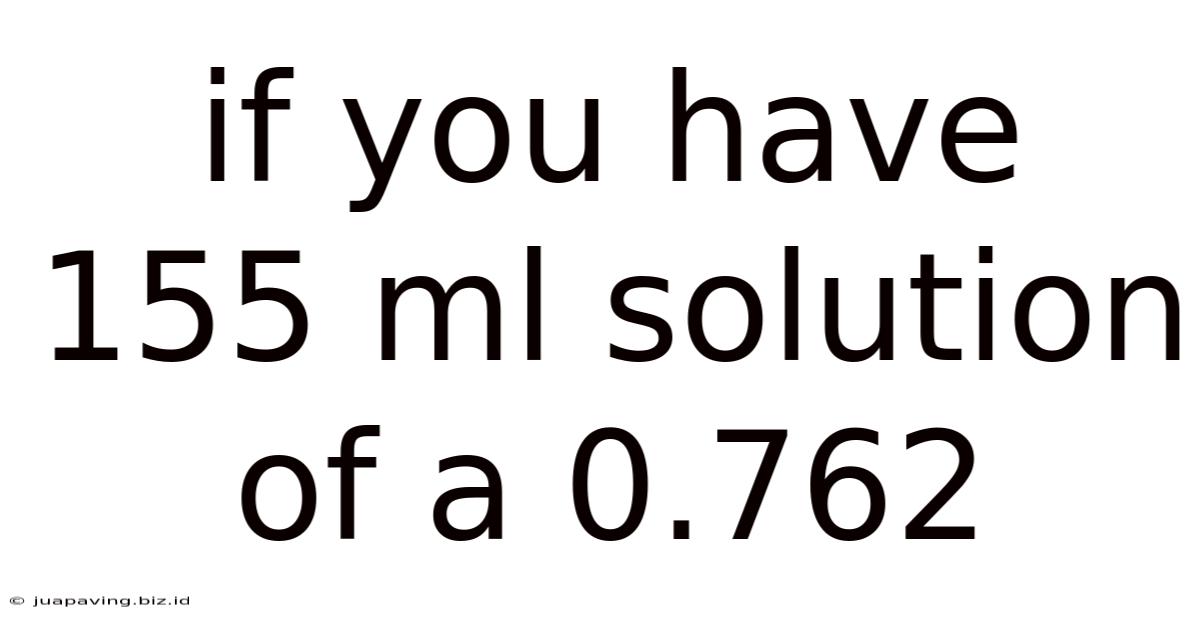
Table of Contents
Unlocking the Secrets of a 155ml, 0.762M Solution: A Deep Dive into Chemistry
This article delves into the world of chemistry, specifically exploring the properties and potential applications of a 155ml solution with a concentration of 0.762M. We will dissect the meaning of molarity, explore how to calculate the number of moles present, and discuss the significance of concentration in various chemical processes. We'll even touch upon the practical implications and potential applications of such a solution, considering different scenarios and the importance of precise measurements in chemistry.
Understanding Molarity (M)
Before we dive into the specifics of our 155ml, 0.762M solution, let's establish a firm understanding of molarity (M). Molarity is a crucial concept in chemistry that expresses the concentration of a solute in a solution. It's defined as the number of moles of solute per liter of solution. The formula for molarity is:
Molarity (M) = Moles of solute / Liters of solution
This simple yet powerful equation allows us to determine the amount of solute present in a given volume of solution, and vice-versa. The unit of molarity is moles per liter (mol/L), often represented simply as M. A 1M solution contains one mole of solute per liter of solution, a 0.5M solution contains half a mole per liter, and so on.
Calculations for Our 155ml, 0.762M Solution
Now, let's apply this understanding to our specific solution: 155ml of a 0.762M solution. Our first step involves converting the volume from milliliters to liters, since molarity uses liters as its volume unit:
155 ml * (1 L / 1000 ml) = 0.155 L
Now we can use the molarity formula to calculate the number of moles of solute present:
Moles of solute = Molarity * Liters of solution
Moles of solute = 0.762 M * 0.155 L = 0.118 moles
Therefore, our 155ml, 0.762M solution contains 0.118 moles of solute.
The Importance of Precise Measurements
The accuracy of our calculations hinges on the precision of the measurements. Inaccurate volume measurements, for example, will directly impact the calculated number of moles. This highlights the critical role of precise measurement techniques in chemistry, including the use of calibrated glassware and careful handling of chemicals. Even small errors in measurement can lead to significant discrepancies in experimental results. Therefore, meticulous attention to detail is crucial when working with chemical solutions.
Factors Affecting Concentration
Several factors influence the concentration of a solution. These include:
-
Temperature: Temperature changes can affect the solubility of the solute, thus altering the concentration. Generally, solubility increases with temperature, although there are exceptions.
-
Pressure: For gaseous solutes, pressure plays a significant role. Increased pressure typically leads to increased solubility and therefore higher concentration.
-
Nature of the solute and solvent: The chemical properties of the solute and solvent determine how readily the solute dissolves in the solvent. Polar solvents, for instance, tend to dissolve polar solutes more effectively than nonpolar solutes.
Applications of Solutions with Defined Concentrations
Solutions with precisely defined concentrations, like our 155ml, 0.762M solution, find extensive use across various scientific disciplines and industrial processes. Some examples include:
-
Titrations: In analytical chemistry, titrations rely on solutions of known concentrations to determine the concentration of an unknown solution. Our solution could serve as a standard solution in such titrations.
-
Spectrophotometry: Spectrophotometry uses the absorbance or transmittance of light to measure the concentration of a solution. Precisely known concentrations are essential for creating calibration curves used in this technique.
-
Pharmaceutical Industry: Accurate concentrations are paramount in pharmaceutical preparations. Solutions with precisely defined concentrations are crucial for producing medications with the correct dosage.
-
Chemical Synthesis: Many chemical reactions require precise concentrations of reactants for optimal yields and control over reaction pathways. Our solution could be a reactant in various chemical syntheses.
Determining the Identity of the Solute
Knowing the concentration (0.762M) and volume (155ml) alone doesn’t tell us the identity of the solute. To determine the solute, we need additional information, such as:
-
Qualitative Analysis: Performing tests to determine the chemical properties (e.g., color, odor, reactivity) of the solute can provide clues to its identity.
-
Quantitative Analysis: Techniques like chromatography or spectroscopy can provide more definitive identification by analyzing the chemical composition of the solute.
Without this extra information, we can only work with the known concentration and volume. This limitation highlights the importance of complete experimental data for accurate characterization of a chemical solution.
Safety Precautions When Handling Chemical Solutions
When working with chemical solutions, especially those with defined concentrations, adhering to safety protocols is non-negotiable. These precautions include:
-
Personal Protective Equipment (PPE): Always wear appropriate PPE such as safety goggles, gloves, and lab coats to protect yourself from potential hazards.
-
Proper Ventilation: Ensure adequate ventilation in the work area to prevent exposure to harmful fumes or vapors.
-
Waste Disposal: Dispose of chemical waste according to established safety procedures to prevent environmental contamination.
-
Emergency Preparedness: Be aware of emergency procedures and have access to safety equipment such as eyewash stations and safety showers.
Advanced Concepts and Further Exploration
The principles discussed here provide a foundation for understanding chemical solutions and their applications. Further exploration could involve:
-
Dilution Calculations: Learning how to calculate the concentration of a solution after dilution.
-
Colligative Properties: Exploring how properties of solutions, such as boiling point elevation and freezing point depression, are affected by solute concentration.
-
Solubility Equilibrium: Understanding the factors governing the solubility of different substances in different solvents.
Conclusion: The Significance of Precise Chemical Knowledge
A 155ml, 0.762M solution, seemingly simple, encapsulates the essence of precision and accuracy in chemistry. Understanding molarity, calculating the number of moles, and appreciating the importance of precise measurements are fundamental to numerous chemical processes. This understanding extends to various applications, from analytical chemistry to the pharmaceutical industry. By combining careful experimentation, accurate calculations, and diligent adherence to safety procedures, we can effectively harness the power of precisely defined chemical solutions. Further exploration into the intricacies of chemical solutions will undoubtedly enrich our understanding of the natural world and its applications in various fields. The seemingly simple solution holds a world of possibilities waiting to be explored, highlighting the crucial role of precision and understanding in the field of chemistry.
Latest Posts
Latest Posts
-
Blood Types And Donation Possibilities Worksheet A
May 31, 2025
-
Which Of The Following Statements About Cholesterol Is False
May 31, 2025
-
Alls Fair In Love And War Shakespeare
May 31, 2025
-
Windows 11 Is The Foundation Software For All Except
May 31, 2025
-
I Know Why The Caged Bird Sings Summary Chapter 1
May 31, 2025
Related Post
Thank you for visiting our website which covers about If You Have 155 Ml Solution Of A 0.762 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.