If An Atom Gains An Electron It Becomes
Juapaving
May 10, 2025 · 7 min read
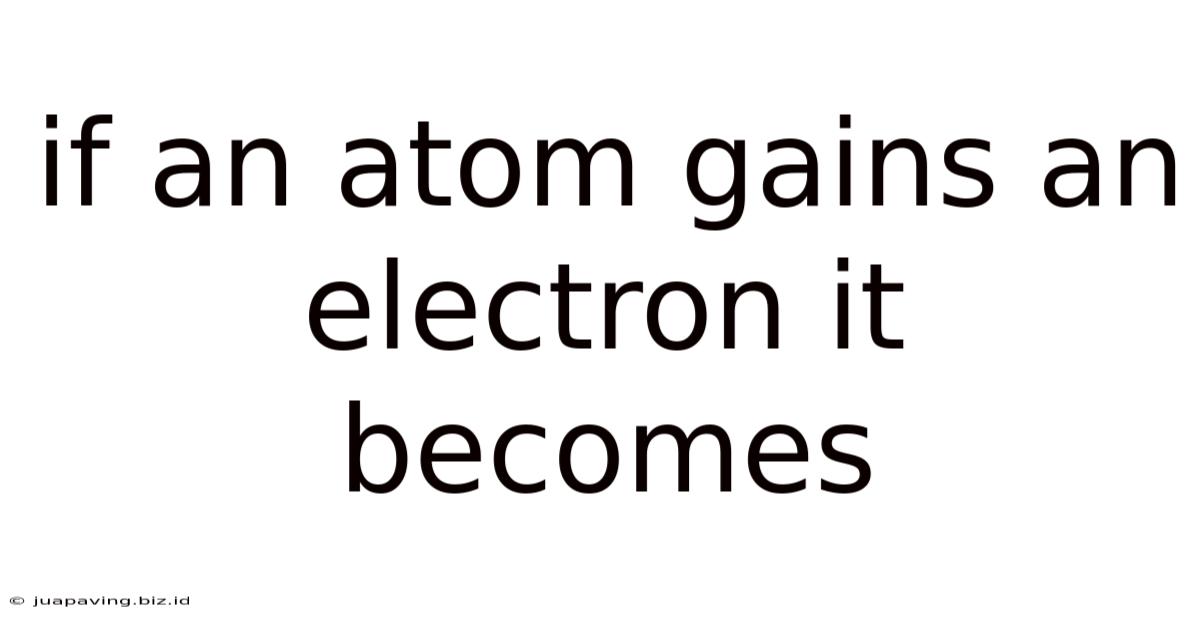
Table of Contents
If an Atom Gains an Electron, It Becomes... a Negative Ion! Understanding Ionization
When an atom gains an electron, it undergoes a fundamental change in its electrical charge, transforming into a negative ion, also known as an anion. This process is a crucial aspect of chemistry and physics, impacting various phenomena from chemical bonding to electrical conductivity. Understanding this transformation requires a grasp of basic atomic structure and the principles governing electron behavior. This article will delve deep into the consequences of an atom gaining an electron, exploring the resulting properties and its implications across various scientific fields.
Atomic Structure: The Foundation of Ionization
Before exploring what happens when an atom gains an electron, let's review the basics of atomic structure. Atoms consist of a central nucleus, containing positively charged protons and neutral neutrons, surrounded by negatively charged electrons orbiting in energy levels or shells. The number of protons defines the atom's atomic number and determines its elemental identity. In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
The Role of Electrons in Chemical Behavior
Electrons, particularly those in the outermost shell (valence electrons), are responsible for an atom's chemical behavior. These valence electrons participate in chemical bonds with other atoms, forming molecules and compounds. The stability of an atom is directly related to the arrangement of its electrons. Atoms tend to gain, lose, or share electrons to achieve a stable electron configuration, often resembling that of a noble gas with a full outer electron shell. This is the driving force behind many chemical reactions.
The Transformation: From Neutral Atom to Negative Ion
When a neutral atom gains an electron, it acquires an extra negative charge. This imbalance between the number of protons (positive charges) and electrons (negative charges) results in a net negative charge on the atom. This negatively charged atom is now called a negative ion or anion. The process of an atom gaining an electron is called reduction.
Understanding the Forces at Play
The addition of an electron isn't a random event. Several factors influence an atom's ability to gain an electron:
-
Electronegativity: Atoms with high electronegativity have a strong attraction for electrons. These atoms are more likely to gain electrons and form negative ions. Elements like fluorine, oxygen, and chlorine are highly electronegative and readily form anions.
-
Electron Affinity: Electron affinity measures the energy change when an atom gains an electron. A high electron affinity indicates that the atom releases a significant amount of energy when it accepts an electron, making the process energetically favorable.
-
Ionization Energy: Although we're focusing on gaining electrons, the ionization energy (the energy required to remove an electron) indirectly plays a role. Atoms with low ionization energy tend to lose electrons easily, leaving behind a positively charged ion (cation), which can subsequently influence the surrounding environment and make gaining an electron by another atom more likely.
Properties of Negative Ions (Anions)
The properties of an atom significantly change upon gaining an electron. These changes stem primarily from the altered charge distribution and electron configuration.
Change in Electrical Charge:
The most significant change is the acquisition of a negative charge. This charge influences the ion's interaction with other charged particles and electric fields. Anions are attracted to positively charged particles and repelled by negatively charged particles.
Change in Size:
Generally, anions are larger than their neutral atom counterparts. This is because the added electron increases electron-electron repulsion, causing the electron cloud to expand. The increased size affects the ion's reactivity and its ability to participate in chemical reactions.
Change in Chemical Reactivity:
The change in electron configuration alters the anion's chemical reactivity. The added electron may fill a previously incomplete electron shell, leading to increased stability and decreased reactivity. However, in some cases, the added electron can destabilize the atom, making it more reactive.
Formation of Ionic Compounds:
Anions frequently participate in the formation of ionic compounds. These compounds are formed through the electrostatic attraction between oppositely charged ions – anions and cations. The strong electrostatic forces hold the ions together in a crystal lattice structure. Table salt (NaCl) is a classic example of an ionic compound, formed from the combination of sodium cations (Na⁺) and chloride anions (Cl⁻).
Examples of Negative Ion Formation
Let's look at some specific examples of how atoms become negative ions:
-
Chlorine (Cl) to Chloride (Cl⁻): Chlorine atoms readily gain one electron to achieve a stable octet (eight electrons) in their outermost shell, forming the chloride anion. This is a very common process in many chemical reactions.
-
Oxygen (O) to Oxide (O²⁻): Oxygen atoms typically gain two electrons to achieve a stable octet, forming the oxide anion. This is fundamental to the formation of many metal oxides.
-
Sulfur (S) to Sulfide (S²⁻): Similar to oxygen, sulfur atoms commonly gain two electrons to form the sulfide anion. This anion is crucial in various sulfide minerals and compounds.
Implications Across Scientific Fields
The formation of negative ions has significant implications in diverse scientific fields:
Chemistry:
Anion formation is central to numerous chemical reactions and the formation of compounds. Understanding the behavior of anions is crucial for predicting reaction pathways and the properties of resulting substances. The study of anions is essential in fields like organic chemistry, inorganic chemistry, and analytical chemistry.
Physics:
Negative ions play a significant role in plasma physics and materials science. In plasmas, the presence of negative ions affects electrical conductivity and plasma stability. Negative ions are also utilized in various technological applications, including ion implantation in semiconductor manufacturing.
Biology and Medicine:
Anions are essential components of biological systems. Many biological molecules, such as phosphate ions (PO₄³⁻) and carbonate ions (CO₃²⁻), are anions. These ions play crucial roles in metabolic processes, maintaining electrolyte balance, and signaling pathways within cells. Moreover, some anions have therapeutic applications in medicine, while others contribute to certain diseases.
Environmental Science:
The presence and behavior of anions in the environment are important considerations. For example, the presence of certain anions in water can impact water quality and ecosystem health. The understanding of anion mobility and reactivity is essential for assessing and mitigating environmental pollution.
Beyond Simple Anion Formation: Complex Anions and Polyatomic Ions
The discussion so far has focused on the formation of simple, monatomic anions from single atoms. However, many anions are polyatomic, meaning they consist of multiple atoms covalently bonded together carrying a net negative charge. These complex ions have their own unique properties and reactivity. Examples include:
-
Hydroxide (OH⁻): This ion is a crucial component in many bases and plays a key role in acid-base reactions.
-
Sulfate (SO₄²⁻): A common anion found in various salts and minerals, with important roles in industrial processes and biological systems.
-
Nitrate (NO₃⁻): A crucial nutrient for plants and found in fertilizers, also having implications for water pollution.
-
Phosphate (PO₄³⁻): Essential for life, participating in energy transfer and the formation of DNA and RNA.
The formation and behavior of these polyatomic anions are equally important as monatomic anions and contribute significantly to the complexity and richness of chemical interactions. Understanding their properties is crucial for numerous applications across many scientific domains.
Conclusion: The Significance of Negative Ion Formation
The process of an atom gaining an electron to become a negative ion is a fundamental process in chemistry and physics. It's a cornerstone concept that underpins our understanding of chemical bonding, reactivity, and the behavior of matter at the atomic level. From the formation of simple ionic compounds to the intricate roles of polyatomic anions in biological systems and industrial processes, the consequences of an atom gaining an electron are far-reaching and profoundly influence the world around us. The continued investigation and deeper understanding of anion formation and behavior will undoubtedly unlock new possibilities and advancements across numerous scientific and technological fields.
Latest Posts
Latest Posts
-
The Bottom Number In A Fraction
May 10, 2025
-
How Many Feet Are In 13 Yards
May 10, 2025
-
What Is The Boiling Point On The Kelvin Scale
May 10, 2025
-
How Many Carbon Atoms Are In The Longest Chain
May 10, 2025
-
Which Substance Cannot Be Separated Physically Or Chemically
May 10, 2025
Related Post
Thank you for visiting our website which covers about If An Atom Gains An Electron It Becomes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.