How To Separate Sugar And Water
Juapaving
Apr 06, 2025 · 5 min read
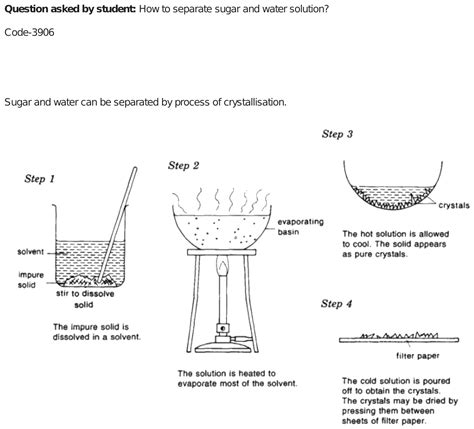
Table of Contents
How to Separate Sugar and Water: A Comprehensive Guide
Separating sugar and water might seem like a simple task, but understanding the underlying principles and choosing the right method can significantly impact efficiency and outcome. This comprehensive guide explores various techniques to separate these two substances, delving into the science behind each method and offering practical advice for achieving optimal results. Whether you're a student tackling a science project or a curious individual exploring the wonders of chemistry, this guide provides a thorough understanding of this common separation challenge.
Understanding the Sugar-Water Mixture
Before diving into separation methods, it's crucial to understand the nature of the sugar-water mixture. Sugar (sucrose) is a soluble solid, meaning it dissolves completely in water to form a homogeneous solution. This means the sugar molecules are evenly distributed throughout the water, making simple physical separation methods like filtration ineffective. To successfully separate them, we need techniques that exploit the differences in their physical properties, such as boiling point and volatility.
Methods for Separating Sugar and Water
Several techniques can effectively separate sugar and water. The optimal choice depends on factors like the quantity of the mixture, the desired purity of the separated components, and the available resources. Let's explore the most common methods:
1. Evaporation
Evaporation is the most straightforward method for separating sugar and water. It leverages the difference in boiling points: water boils at 100°C (212°F) at standard atmospheric pressure, while sugar has a much higher decomposition temperature.
Procedure:
- Gentle Heating: Carefully heat the sugar-water solution in a suitable container, like a beaker or saucepan. Avoid rapid boiling, which can cause the sugar to caramelize and potentially burn.
- Water Vaporization: As the solution heats, the water will evaporate, turning into water vapor.
- Sugar Crystallization: As the water evaporates, the sugar concentration increases until the solution becomes saturated. Eventually, the sugar will begin to crystallize and precipitate out of the solution.
- Crystal Collection: Once most of the water has evaporated, you'll be left with solid sugar crystals. You can carefully scrape them from the container.
Advantages:
- Simplicity: It's a relatively simple and easy-to-understand technique.
- Effectiveness: It effectively separates the sugar and water, provided you are patient and avoid overheating.
Disadvantages:
- Time-Consuming: Evaporation is a slow process, requiring significant time to complete.
- Potential for Sugar Degradation: Overheating can cause the sugar to caramelize or even burn, impacting the purity of the separated sugar.
2. Distillation
Distillation is a more advanced technique that utilizes the difference in boiling points to separate the components of a liquid mixture. It's a more efficient method than simple evaporation, particularly for large quantities of the mixture.
Procedure:
- Heating and Vaporization: The sugar-water solution is heated in a distillation flask. The water vaporizes first, as it has a lower boiling point.
- Condensation: The water vapor rises through a condenser, which cools it back into liquid form.
- Collection: The condensed water is collected in a separate container, leaving the sugar behind in the distillation flask.
Advantages:
- Efficiency: Distillation is significantly faster and more efficient than simple evaporation.
- High Purity: It yields a higher purity of water than evaporation.
Disadvantages:
- Complexity: Requires more specialized equipment compared to evaporation.
- Cost: The equipment needed for distillation can be expensive.
3. Reverse Osmosis
Reverse osmosis is a membrane-based separation technique that uses pressure to force water molecules through a semi-permeable membrane. The membrane allows water molecules to pass, but it prevents larger sugar molecules from passing through.
Procedure:
- Pressure Application: High pressure is applied to the sugar-water solution.
- Water Separation: The water molecules pass through the membrane, while the sugar molecules are retained.
- Concentrate Collection: The sugar-rich solution remains on one side of the membrane.
Advantages:
- High Purity of Water: Reverse osmosis produces highly purified water.
- Energy Efficiency (in some cases): Depending on the system, it can be more energy-efficient than distillation for certain scales.
Disadvantages:
- High Initial Cost: Reverse osmosis systems can be expensive to purchase and maintain.
- Membrane Fouling: The membrane can become clogged over time, reducing efficiency.
- Not Ideal for Large-Scale Separation of Sugar: It's less effective for recovering large amounts of sugar, more focused on purifying the water.
4. Chromatography (Less Practical for this Application)
While chromatography is a powerful separation technique, it is less practical for separating sugar and water on a simple scale. Chromatography relies on the differential affinity of substances for a stationary and mobile phase. While it could be adapted, it's far less efficient than evaporation or distillation for this specific separation.
Choosing the Right Method
The optimal method for separating sugar and water depends on several factors:
- Scale of Separation: For small quantities, evaporation is often sufficient. For large quantities, distillation might be more efficient.
- Desired Purity: If high-purity water is the goal, distillation or reverse osmosis are preferred.
- Available Resources: The choice of method will depend on the equipment and resources available.
Safety Precautions
When performing any of these separation techniques, it's crucial to follow safety precautions:
- Heat Safety: Always use appropriate heat-resistant gloves and glassware when handling hot solutions.
- Sharp Objects: Be cautious when handling sharp instruments, such as knives or spatulas, for scraping sugar crystals.
- Electrical Safety: If using electrical equipment, ensure it is properly grounded and used in a safe environment.
Conclusion
Separating sugar and water involves exploiting the differences in their physical properties. Several methods exist, each with its advantages and disadvantages. By understanding the underlying principles and selecting the appropriate technique, you can efficiently and safely separate these two substances. Remember to prioritize safety and carefully consider the scale of your separation and desired purity of the separated components when choosing a method. This guide provides a comprehensive overview to empower you to tackle this common separation challenge with confidence and success.
Latest Posts
Latest Posts
-
Which Of The Following Is An Example Of A Compound
Apr 08, 2025
-
Solving Linear Equations With One Variable
Apr 08, 2025
-
Approximately Two Thirds Of Indias Gdp Is Made Up Of
Apr 08, 2025
-
Label Features Of An Animal Cell
Apr 08, 2025
-
Region Around The Nucleus Where The Electrons Are Found
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How To Separate Sugar And Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.