How To Find The Mole Of Something
Juapaving
Apr 08, 2025 · 5 min read
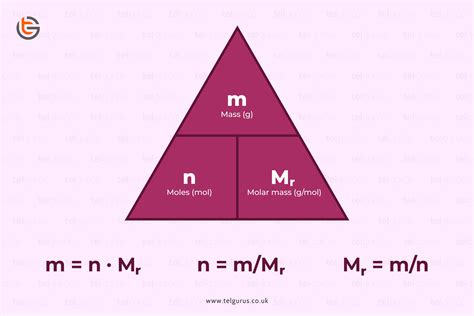
Table of Contents
How to Find the Mole of Something: A Comprehensive Guide
The mole (mol) is a fundamental unit in chemistry, representing a specific number of particles—6.022 x 10²³ to be exact, also known as Avogadro's number. Understanding how to calculate the mole is crucial for anyone studying chemistry, as it's the cornerstone of stoichiometry and many other chemical calculations. This comprehensive guide will walk you through various methods of determining the mole of a substance, from simple calculations to more complex scenarios.
Understanding the Mole Concept
Before diving into calculations, let's solidify the understanding of what a mole actually represents. Imagine you have a dozen eggs. You instantly know you have 12 eggs. Similarly, a mole represents a specific, incredibly large number of particles (atoms, molecules, ions, etc.). This standardized number allows chemists to compare and work with different substances on a consistent basis.
Key takeaway: The mole is a counting unit, just like a dozen, but on a much grander scale, providing a bridge between the macroscopic world (grams) and the microscopic world (atoms and molecules).
Calculating Moles from Mass
One of the most common ways to determine the number of moles is using the substance's mass and its molar mass. Molar mass is the mass of one mole of a substance, usually expressed in grams per mole (g/mol). You can find molar mass by adding up the atomic masses of all the atoms in a molecule, using the periodic table as your reference.
Formula:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
Example 1: Finding Moles of Water
Let's say you have 18 grams of water (H₂O). The molar mass of water is calculated as follows:
- Hydrogen (H) has an atomic mass of approximately 1 g/mol, and there are two hydrogen atoms.
- Oxygen (O) has an atomic mass of approximately 16 g/mol.
Therefore, the molar mass of H₂O = (2 * 1 g/mol) + 16 g/mol = 18 g/mol.
Now, we can calculate the number of moles:
Moles = 18 g / 18 g/mol = 1 mol
Example 2: Finding Moles of Sodium Chloride
You have 58.5 grams of sodium chloride (NaCl). The molar mass of NaCl:
- Sodium (Na) has an atomic mass of approximately 23 g/mol.
- Chlorine (Cl) has an atomic mass of approximately 35.5 g/mol.
Therefore, the molar mass of NaCl = 23 g/mol + 35.5 g/mol = 58.5 g/mol.
Moles = 58.5 g / 58.5 g/mol = 1 mol
Calculating Moles from Number of Particles
Alternatively, if you know the number of particles (atoms, molecules, ions, etc.) you can directly calculate the number of moles using Avogadro's number.
Formula:
Moles (mol) = Number of Particles / Avogadro's Number (6.022 x 10²³ particles/mol)
Example 3: Finding Moles from Atoms
You have 1.2044 x 10²⁴ atoms of carbon (C). To find the number of moles:
Moles = 1.2044 x 10²⁴ atoms / 6.022 x 10²³ atoms/mol = 2 mol
Example 4: Finding Moles from Molecules
You have 3.011 x 10²³ molecules of oxygen (O₂). To find the number of moles:
Moles = 3.011 x 10²³ molecules / 6.022 x 10²³ molecules/mol = 0.5 mol
Calculating Moles in Solutions
When dealing with solutions (a solute dissolved in a solvent), calculating moles involves using molarity. Molarity (M) is defined as the number of moles of solute per liter of solution.
Formula:
Moles (mol) = Molarity (mol/L) x Volume (L)
Example 5: Finding Moles in a Solution
You have 250 mL of a 2.0 M solution of sodium hydroxide (NaOH). First, convert the volume to liters: 250 mL = 0.25 L.
Moles = 2.0 mol/L x 0.25 L = 0.5 mol
Calculating Moles in Gas
For gases, the ideal gas law provides a method for calculating the number of moles. The ideal gas law is PV = nRT, where:
- P = pressure (usually in atmospheres, atm)
- V = volume (usually in liters, L)
- n = number of moles (mol)
- R = ideal gas constant (0.0821 L·atm/mol·K)
- T = temperature (in Kelvin, K)
Formula (solving for n):
Moles (n) = PV / RT
Example 6: Finding Moles of a Gas
A gas occupies a volume of 5.6 L at a pressure of 1 atm and a temperature of 273 K. Using the ideal gas law:
Moles = (1 atm * 5.6 L) / (0.0821 L·atm/mol·K * 273 K) ≈ 0.25 mol
Dealing with More Complex Scenarios
Many chemical reactions involve multiple reactants and products. Stoichiometry uses mole calculations to determine the quantitative relationships between reactants and products. This often involves using balanced chemical equations to determine mole ratios.
Example 7: Stoichiometry Calculation
Consider the balanced chemical equation for the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
If you have 1 mole of methane (CH₄), the balanced equation tells us you'll need 2 moles of oxygen (O₂) to completely react, and you'll produce 1 mole of carbon dioxide (CO₂) and 2 moles of water (H₂O).
Example 8: Limiting Reactants
In reactions with multiple reactants, one reactant may be completely consumed before others. This reactant is called the limiting reactant, and it determines the maximum amount of product that can be formed. Determining the limiting reactant involves calculating the moles of each reactant and comparing them according to the stoichiometric ratios from the balanced chemical equation.
Practical Applications of Mole Calculations
The mole concept is essential in numerous fields:
- Analytical Chemistry: Determining the concentration of substances in solutions or samples.
- Pharmaceutical Chemistry: Calculating drug dosages and formulations.
- Environmental Chemistry: Measuring pollutant concentrations in air and water.
- Industrial Chemistry: Optimizing chemical reactions and production processes.
Conclusion
Mastering mole calculations is paramount for success in chemistry and related fields. This guide has covered various methods for determining the number of moles, ranging from simple mass-based calculations to more complex scenarios involving solutions, gases, and stoichiometry. Remember to always use the appropriate formula based on the given information and always double-check your units for consistency. With practice and a solid understanding of the fundamental principles, you'll confidently navigate the world of moles and unlock a deeper understanding of chemical quantities.
Latest Posts
Latest Posts
-
What Do You Call A Group Of Lions
Apr 08, 2025
-
Whats The Lcm Of 9 And 12
Apr 08, 2025
-
Which Is Not One Of The Five Pillars Of Islam
Apr 08, 2025
-
How Many Feet Is 65 In
Apr 08, 2025
-
5 Letter Words Beginning With Ae
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about How To Find The Mole Of Something . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.