How To Find Relative Abundance Of Isotopes
Juapaving
Mar 12, 2025 · 6 min read
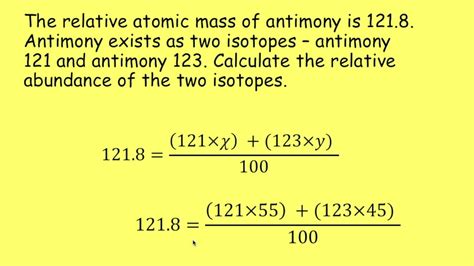
Table of Contents
How to Find the Relative Abundance of Isotopes: A Comprehensive Guide
Determining the relative abundance of isotopes is crucial in various fields, from geology and archaeology to medicine and nuclear physics. Isotopes, atoms of the same element with varying neutron numbers, possess distinct properties that influence their behavior in different systems. Understanding their relative abundance allows us to unravel the history of materials, diagnose medical conditions, and even understand the processes that shaped our planet. This comprehensive guide will walk you through various methods for determining the relative abundance of isotopes.
Understanding Isotopes and Relative Abundance
Before diving into the methods, let's establish a solid foundation. Isotopes are atoms of the same element that share the same number of protons but differ in the number of neutrons. This difference affects their mass, but not their chemical properties significantly. Each element has a variety of isotopes, some stable and some radioactive.
Relative abundance refers to the proportion of each isotope of an element present in a naturally occurring sample. It's typically expressed as a percentage. For example, carbon has two main stable isotopes: ¹²C (carbon-12) and ¹³C (carbon-13). The relative abundance of ¹²C is approximately 98.9%, while ¹³C accounts for about 1.1%. This means that in a typical sample of carbon, about 98.9 out of every 100 carbon atoms will be ¹²C.
The relative abundance of isotopes can vary depending on the source of the sample. Factors like geological processes, cosmic ray bombardment, and even biological processes can influence isotopic ratios. This variation is what makes isotopic analysis a powerful tool for scientific investigation.
Methods for Determining Relative Abundance of Isotopes
Several sophisticated techniques are used to determine the relative abundance of isotopes. These methods rely on the differences in mass between the isotopes, which allows for their separation and quantification.
1. Mass Spectrometry: The Workhorse of Isotope Analysis
Mass spectrometry (MS) is the most widely used technique for determining isotopic relative abundance. It works on the principle of separating ions based on their mass-to-charge ratio (m/z). The process typically involves these steps:
- Ionization: The sample is first ionized, converting neutral atoms into charged ions. This can be achieved through various methods, such as electron ionization, electrospray ionization, or laser ablation.
- Acceleration: The ions are then accelerated using an electric field, giving them kinetic energy.
- Separation: The accelerated ions are passed through a magnetic field, which deflects them based on their m/z ratio. Lighter ions are deflected more than heavier ions.
- Detection: A detector measures the abundance of each ion, providing a mass spectrum that shows the relative abundance of different isotopes.
Different types of mass spectrometers exist, each with its own strengths and weaknesses. Gas chromatography-mass spectrometry (GC-MS) combines gas chromatography (separation of volatile compounds) with mass spectrometry for analyzing complex mixtures. Inductively coupled plasma mass spectrometry (ICP-MS) is excellent for analyzing trace elements in various matrices. Thermal ionization mass spectrometry (TIMS) is known for its high precision and accuracy, particularly for long-lived radioactive isotopes.
Advantages of Mass Spectrometry:
- High precision and accuracy: MS can measure isotopic ratios with very high precision, often down to parts per million or even parts per billion.
- Versatility: It can analyze a wide range of elements and samples.
- Sensitivity: It can detect even trace amounts of isotopes.
Limitations of Mass Spectrometry:
- Cost: Mass spectrometers are expensive instruments.
- Complexity: The operation and maintenance of MS instruments require specialized training and expertise.
- Sample preparation: Sample preparation can be time-consuming and may require specialized techniques.
2. Nuclear Magnetic Resonance (NMR) Spectroscopy
Nuclear Magnetic Resonance (NMR) spectroscopy is another powerful technique, particularly useful for analyzing isotopes with non-zero nuclear spin. NMR exploits the interaction of nuclear spins with a magnetic field. Different isotopes have different nuclear spins and gyromagnetic ratios, leading to distinct NMR signals. By analyzing the intensity of these signals, the relative abundance of isotopes can be determined.
Advantages of NMR Spectroscopy:
- Non-destructive: The sample is not consumed during the analysis.
- High resolution: NMR can provide detailed information about the chemical environment of the isotopes.
- Versatility: It can be applied to a variety of samples, including solids, liquids, and gases.
Limitations of NMR Spectroscopy:
- Sensitivity: NMR is generally less sensitive than mass spectrometry.
- Sample requirements: NMR often requires relatively large sample quantities.
- Specialized expertise: Interpretation of NMR spectra requires specialized knowledge.
3. Isotope Ratio Mass Spectrometry (IRMS)
Isotope Ratio Mass Spectrometry (IRMS) is a specialized type of mass spectrometry specifically designed for high-precision measurement of isotopic ratios. It is frequently employed in fields such as environmental science, archaeology, and geochemistry to determine the relative abundances of isotopes, particularly for stable isotopes like carbon-13/carbon-12, nitrogen-15/nitrogen-14, and oxygen-18/oxygen-16. IRMS often incorporates sophisticated techniques to minimize systematic errors and achieve high accuracy.
Advantages of IRMS:
- High precision: Designed for precise isotope ratio measurements.
- Wide range of applications: Used across various scientific disciplines.
- Suitable for stable isotope analysis: Ideal for determining relative abundance of stable isotopes.
Limitations of IRMS:
- Cost: High initial investment and ongoing maintenance costs.
- Specialized expertise: Requires specialized knowledge and training.
- Sample preparation: Requires careful sample preparation to avoid contamination.
4. Other Methods
While mass spectrometry and NMR are the dominant techniques, other methods exist for determining isotopic relative abundance in specific scenarios. These include:
- Activation analysis: This technique involves bombarding the sample with neutrons or other particles, making some isotopes radioactive. The subsequent decay characteristics can be used to determine isotopic ratios.
- Spectroscopic methods: Certain spectroscopic techniques, like atomic absorption spectroscopy or inductively coupled plasma optical emission spectrometry (ICP-OES), can be used to indirectly infer isotopic ratios based on spectral line shifts.
Applications of Isotope Abundance Measurements
The determination of relative isotopic abundance has far-reaching implications across multiple scientific disciplines. Here are some key applications:
- Geochronology: Determining the age of geological samples using radioactive isotopes like uranium and lead.
- Archaeology: Tracing the origins of artifacts and materials by analyzing their isotopic composition.
- Paleoclimatology: Reconstructing past climates by studying the isotopic ratios in ice cores, sediments, and other archives.
- Forensic science: Identifying the source of materials involved in criminal investigations.
- Medicine: Diagnosing certain metabolic disorders and tracking the metabolism of drugs.
- Environmental science: Monitoring pollution sources and studying biogeochemical cycles.
- Nuclear physics: Understanding nuclear reactions and decay processes.
Conclusion
Determining the relative abundance of isotopes is a cornerstone of many scientific investigations. Mass spectrometry, particularly in its various forms (e.g., GC-MS, ICP-MS, TIMS, IRMS), remains the workhorse technique due to its precision, versatility, and sensitivity. NMR spectroscopy offers a complementary approach, especially for isotopes with nuclear spin. Understanding the strengths and limitations of each method, along with proper sample preparation, is critical for accurate and reliable results. The broad applicability of isotope ratio measurements across multiple fields underscores its crucial role in advancing our understanding of the world around us. Further developments in instrumentation and analytical techniques promise even greater precision and sensitivity in the future, opening up new possibilities for scientific exploration.
Latest Posts
Latest Posts
-
The Male Accessory Glands Include The
May 09, 2025
-
The Starting Molecule For Glycolysis Is
May 09, 2025
-
How Many Months In Three Years
May 09, 2025
-
Is 19 A Prime Number Or A Composite Number
May 09, 2025
-
Is Force Increase On An Inclined Plane
May 09, 2025
Related Post
Thank you for visiting our website which covers about How To Find Relative Abundance Of Isotopes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.