How Many Valence Electrons In Iron
Juapaving
Apr 06, 2025 · 6 min read
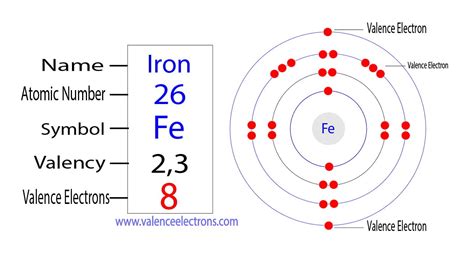
Table of Contents
How Many Valence Electrons Does Iron Have? Understanding Electron Configuration and Chemical Behavior
Iron, a ubiquitous element crucial for life and industry, presents an interesting case study in understanding valence electrons and their implications for chemical bonding and reactivity. While the answer to the question "How many valence electrons does iron have?" might seem straightforward, a deeper dive reveals nuances related to electron configuration, oxidation states, and the complexities of transition metals. This article will explore these aspects comprehensively.
What are Valence Electrons?
Before delving into the specifics of iron, let's establish a clear understanding of valence electrons. These are the electrons located in the outermost shell (or energy level) of an atom. They are the primary players in chemical bonding, determining how an atom interacts with other atoms to form molecules and compounds. The number of valence electrons dictates an element's reactivity and the types of bonds it can form – ionic, covalent, or metallic. Atoms strive to achieve a stable electron configuration, often by gaining, losing, or sharing valence electrons to attain a full outer shell (typically eight electrons, following the octet rule, with some exceptions).
Iron's Electron Configuration: Unraveling the Mystery
Iron (Fe), with an atomic number of 26, possesses 26 electrons. To determine the number of valence electrons, we need to examine its electron configuration. This configuration describes how electrons are distributed among different energy levels and subshells within the atom. Iron's electron configuration is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶.
This notation might seem daunting at first glance, but it's quite systematic. Each number represents the principal quantum number (energy level), and the letters (s, p, d, f) represent the subshells within each energy level. The superscript numbers indicate the number of electrons in each subshell.
Understanding the Significance of 4s and 3d Orbitals
Now, here's where things get interesting regarding iron's valence electrons. Traditionally, only the outermost electrons are considered valence electrons. In simpler atoms, this is clear-cut. However, transition metals like iron present a more complex scenario. Iron has electrons in both the 4s and 3d orbitals. While the 4s orbital is higher in energy than the 3d orbital in a neutral atom, the energy difference is relatively small. This small difference means that both the 4s and 3d electrons can participate in chemical bonding, blurring the lines when defining valence electrons.
How Many Valence Electrons Does Iron Really Have?
Given iron's electron configuration, a simplistic answer would point to two valence electrons (from the 4s orbital). However, this is an oversimplification. In reality, iron can exhibit variable valency, meaning it can lose different numbers of electrons depending on the chemical environment. This is a hallmark characteristic of transition metals.
The 3d electrons are also readily available for bonding, particularly in the formation of various iron compounds. Therefore, while the precise number is debatable due to the involvement of both 4s and 3d electrons, it's more accurate to say that iron can have multiple valence electrons ranging from two to eight, but most commonly two or three.
Iron's Oxidation States: A Deeper Dive into Valence Electrons
The concept of oxidation state provides a more accurate reflection of iron's varying valence electron participation. The oxidation state represents the apparent charge on an atom if all bonds were completely ionic. Iron commonly exhibits +2 and +3 oxidation states, corresponding to the loss of two or three electrons, respectively.
-
Fe²⁺ (Iron(II) or Ferrous): In this state, iron has lost two electrons, typically from the 4s orbital. It's still considered to have six 3d electrons.
-
Fe³⁺ (Iron(III) or Ferric): Here, iron has lost three electrons – two from the 4s and one from the 3d orbital. It now possesses five 3d electrons.
Other, less common oxidation states, such as +4, +5, and +6, are also possible under specific conditions, further showcasing the variable valency of iron.
The Role of Valence Electrons in Iron's Chemical Behavior
The variable number of valence electrons directly impacts iron's chemical reactivity and the properties of its compounds. This variable valency leads to a wide range of iron compounds with diverse applications.
Examples of Iron Compounds and their Valence Electrons:
-
Iron oxides (FeO, Fe₂O₃, Fe₃O₄): These compounds are prevalent in nature and industrial processes, showcasing different oxidation states (+2, +3) and thus different numbers of effectively available valence electrons.
-
Iron sulfides (FeS, FeS₂): These are important minerals and are involved in various industrial applications. The differing oxidation states of iron determine the specific properties of each sulfide.
-
Iron halides (FeCl₂, FeCl₃): These compounds demonstrate the difference in properties between the +2 and +3 oxidation states of iron, influenced by the number of electrons involved in bonding.
-
Hemoglobin: This crucial protein in blood uses iron in the +2 oxidation state to bind and transport oxygen throughout the body. The two available electrons from the Fe²⁺ ion are vital for this function.
Applications of Iron and its Valence Electron Behavior
Iron's widespread applications are directly linked to its variable valency and chemical behavior. Its use spans various fields:
-
Steel Production: Iron is the primary component of steel, an alloy with crucial structural applications due to its strength and durability. The alloying elements interact with iron's valence electrons, influencing the final material properties.
-
Catalysis: Iron compounds serve as catalysts in various chemical processes, facilitating reactions by interacting with reactants through their valence electrons. The Haber-Bosch process, for ammonia synthesis, is a notable example.
-
Pigments: Iron oxides are used as pigments in paints and other coloring agents, their color varying based on the iron's oxidation state and crystal structure.
-
Medicine: Iron is an essential nutrient for human health, playing a vital role in oxygen transport and various metabolic processes. Iron deficiency anemia arises when there is insufficient iron available to form sufficient hemoglobin.
Conclusion: Understanding Iron's Complexity
While a simple answer to the question "How many valence electrons does iron have?" might seem to be two, the reality is significantly more nuanced. Iron's transition metal nature allows it to exhibit variable valency, meaning it can utilize varying numbers of electrons from both its 4s and 3d orbitals for bonding, leading to a wide range of oxidation states and compound formation. This variability underpins its crucial role in biological systems, industrial processes, and a vast array of applications. Understanding this complexity is key to appreciating iron's significance in the world around us. This comprehensive analysis offers a detailed exploration of iron's electron configuration, oxidation states, and the crucial role of its valence electrons in shaping its diverse chemical behavior and widespread applications.
Latest Posts
Latest Posts
-
What Is A Factor Of 50
Apr 09, 2025
-
How To Turn A Ratio Into A Percentage
Apr 09, 2025
-
Indus Valley Civilization Pdf For Class 6
Apr 09, 2025
-
What Is The Prime Factorization For 180
Apr 09, 2025
-
What Is The Least Reactive Metal
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons In Iron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.