How Many Valence Electrons Does K Have
Juapaving
Mar 10, 2025 · 5 min read
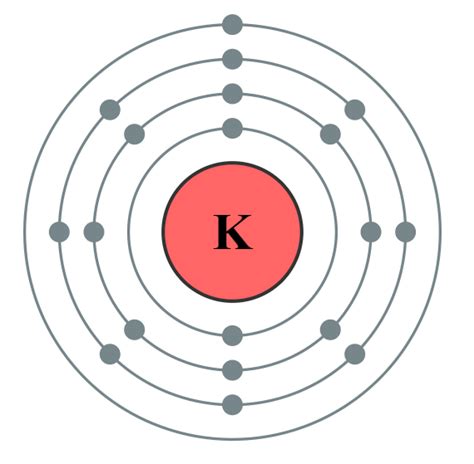
Table of Contents
How Many Valence Electrons Does Potassium (K) Have? A Deep Dive into Atomic Structure and Chemical Behavior
Potassium (K), a crucial element for life, plays a vital role in various biological processes. Understanding its chemical behavior hinges on knowing its electronic configuration and, specifically, the number of valence electrons it possesses. This article delves into the intricacies of potassium's atomic structure to definitively answer the question: how many valence electrons does potassium have? We will explore the concept of valence electrons, their significance in chemical bonding, and how potassium's unique electronic structure influences its reactivity and properties.
Understanding Valence Electrons: The Key to Chemical Bonding
Before we pinpoint the number of valence electrons in potassium, let's establish a firm grasp of what valence electrons are. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. They're the glue that holds atoms together to create molecules and compounds. The number of valence electrons directly influences an element's chemical properties.
The outermost shell is also referred to as the valence shell. Atoms strive to achieve a stable electron configuration, often represented by a full valence shell (typically eight electrons, following the octet rule, although there are exceptions, particularly for elements in the first and second periods). This drive for stability dictates how atoms interact and form chemical bonds.
Determining Potassium's Valence Electrons: Electronic Configuration and Periodicity
To determine the number of valence electrons in potassium (K), we need to examine its electronic configuration. Potassium's atomic number is 19, meaning it has 19 protons and 19 electrons in a neutral atom. The electronic configuration represents the distribution of these electrons across different energy levels or shells. Potassium's electronic configuration is: 1s²2s²2p⁶3s²3p⁶4s¹.
Let's break this down:
- 1s²: Two electrons in the first energy level (n=1)
- 2s²: Two electrons in the second energy level (n=2)
- 2p⁶: Six electrons in the second energy level (n=2)
- 3s²: Two electrons in the third energy level (n=3)
- 3p⁶: Six electrons in the third energy level (n=3)
- 4s¹: One electron in the fourth energy level (n=4)
The outermost shell in potassium's electronic configuration is the fourth energy level (n=4), which contains only one electron in the 4s orbital. Therefore, potassium has only one valence electron.
The Significance of Periodicity: Understanding Group 1 Alkali Metals
Potassium belongs to Group 1 of the periodic table, also known as the alkali metals. Elements within the same group share similar chemical properties because they have the same number of valence electrons. All alkali metals have one valence electron. This commonality explains why potassium, like other alkali metals (lithium, sodium, rubidium, cesium, and francium), exhibits similar reactivity.
The presence of only one valence electron makes alkali metals highly reactive. They readily lose this single electron to achieve a stable octet configuration, forming positively charged ions (cations) with a +1 charge (K⁺). This tendency to lose an electron and form positive ions is a defining characteristic of alkali metals and directly stems from having only one valence electron.
Potassium's Chemical Behavior: A Consequence of its Single Valence Electron
Potassium's single valence electron profoundly influences its chemical behavior:
Reactivity:
- Highly Reactive: Due to its lone valence electron, potassium is extremely reactive. It readily reacts with nonmetals, particularly halogens (like chlorine and bromine), to form ionic compounds. These reactions are often exothermic, releasing significant amounts of energy.
- Formation of Ionic Compounds: Potassium readily loses its valence electron to form a K⁺ ion, achieving a stable electron configuration similar to the noble gas argon. This ionic bonding is a cornerstone of potassium's chemical reactivity.
- Reactions with Water: Potassium reacts vigorously with water, producing hydrogen gas and potassium hydroxide. This reaction is highly exothermic and can even be explosive. The single valence electron is readily donated to the water molecule, initiating the reaction.
Oxidation States:
Potassium almost exclusively exhibits an oxidation state of +1. This reflects its tendency to lose one electron to achieve a stable electron configuration. The +1 oxidation state is consistent with the loss of its single valence electron.
Bonding Characteristics:
Potassium primarily forms ionic bonds. Its tendency to readily lose its valence electron to achieve a stable electron configuration is the driving force behind its ionic bonding. Covalent bonding is less common for potassium because it does not readily share its valence electron.
Applications of Potassium: Leveraging its Chemical Properties
The unique chemical properties of potassium, stemming directly from its single valence electron, make it valuable in various applications:
- Fertilizers: Potassium is a crucial nutrient for plant growth, playing a vital role in numerous plant processes. Potassium-containing fertilizers are widely used in agriculture to enhance crop yields.
- Electrolyte Solutions: Potassium ions (K⁺) are essential electrolytes in biological systems, contributing to nerve impulse transmission, muscle contraction, and fluid balance. Potassium-rich solutions are used in medicine.
- Industrial Applications: Potassium compounds find applications in various industrial processes, including the production of glass, soaps, and other chemicals.
- Food Processing: Potassium salts are added to some foods as preservatives and flavor enhancers.
Conclusion: Understanding Potassium's Reactivity through its Valence Electrons
In conclusion, potassium (K) has one valence electron. This single valence electron is the key to understanding its high reactivity, its tendency to form ionic bonds, and its +1 oxidation state. Its position in Group 1 of the periodic table, as an alkali metal, further reinforces the significance of this single valence electron in dictating its chemical behavior and making it an essential element in diverse applications, from fertilizers to biological systems. By understanding the fundamental concepts of atomic structure and valence electrons, we gain valuable insights into the properties and reactivity of elements like potassium. This knowledge is crucial in various scientific fields, including chemistry, biology, and materials science. The seemingly simple question, "How many valence electrons does potassium have?" opens the door to a deeper understanding of the intricate world of chemical bonding and the remarkable properties of the elements.
Latest Posts
Latest Posts
-
Most Abundant Gas In The Atmosphere
Mar 10, 2025
-
How Many Feet Are 72 Inches
Mar 10, 2025
-
Organisms That Make Their Own Food
Mar 10, 2025
-
Adjectives Beginning With The Letter V
Mar 10, 2025
-
Describe The Features Of The Globe
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does K Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
