How Many Valence Electrons Does Chloride Have
Juapaving
Mar 11, 2025 · 5 min read
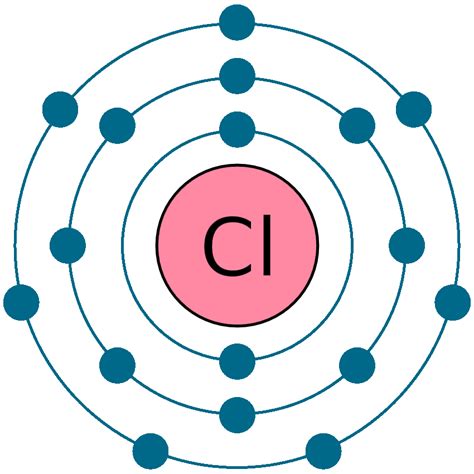
Table of Contents
How Many Valence Electrons Does Chloride Have? A Deep Dive into Atomic Structure and Chemical Bonding
Understanding the number of valence electrons an atom possesses is fundamental to comprehending its chemical behavior and how it interacts with other atoms to form molecules and compounds. This article will delve deep into the question: how many valence electrons does chloride have? We'll explore the underlying principles of atomic structure, electron configuration, and the significance of valence electrons in chemical bonding, particularly focusing on the chloride ion (Cl⁻).
Understanding Atomic Structure and Electron Configuration
Before we determine the number of valence electrons in chloride, let's establish a foundational understanding of atomic structure. An atom consists of a central nucleus containing protons (positively charged particles) and neutrons (neutral particles). Surrounding the nucleus are electrons (negatively charged particles) arranged in energy levels or shells. These shells have different capacities for electrons. The first shell can hold up to two electrons, the second shell up to eight, and so on.
The arrangement of electrons in an atom's shells is described by its electron configuration. This configuration dictates the atom's chemical properties and reactivity. For chlorine (Cl), a neutral atom with an atomic number of 17, the electron configuration is 1s²2s²2p⁶3s²3p⁵. This means:
- 1s²: Two electrons in the first energy level (shell).
- 2s²: Two electrons in the second energy level.
- 2p⁶: Six electrons in the second energy level (p-subshell).
- 3s²: Two electrons in the third energy level.
- 3p⁵: Five electrons in the third energy level (p-subshell).
What are Valence Electrons?
Valence electrons are the electrons located in the outermost shell (the highest energy level) of an atom. These electrons are crucial because they participate directly in chemical bonding. They determine an atom's reactivity and how it will interact with other atoms. Atoms tend to gain, lose, or share valence electrons to achieve a stable electron configuration, typically resembling a noble gas (a group 18 element with a full outermost shell). This stable configuration is often referred to as an octet (eight electrons in the outermost shell), although the first shell only requires two electrons for stability (duet rule).
Determining the Valence Electrons of Chlorine
Looking at chlorine's electron configuration (1s²2s²2p⁶3s²3p⁵), we can identify the valence electrons as those in the outermost shell, which is the third energy level (n=3). This shell contains the 3s and 3p electrons. Therefore, a neutral chlorine atom has 3s² + 3p⁵ = 7 valence electrons.
Chloride Ion (Cl⁻) and its Valence Electrons
Chlorine is highly reactive because it has seven valence electrons, one short of a stable octet. To achieve this stable configuration, chlorine readily gains one electron. When it gains an electron, it becomes a chloride ion (Cl⁻). This process is an example of ionic bonding.
The added electron fills the 3p subshell, completing the octet in the third energy level. The electron configuration of the chloride ion is now 1s²2s²2p⁶3s²3p⁶. Since the outermost shell is now full, the chloride ion has 8 valence electrons.
Significance of Valence Electrons in Chemical Bonding
The number of valence electrons significantly influences the type of chemical bonds an atom forms. There are three main types:
1. Ionic Bonds
Ionic bonds form when atoms transfer electrons. Atoms with a low number of valence electrons (like alkali metals) tend to lose electrons to achieve a stable octet, forming positively charged ions (cations). Atoms with a high number of valence electrons (like halogens) tend to gain electrons to achieve a stable octet, forming negatively charged ions (anions). The electrostatic attraction between these oppositely charged ions forms the ionic bond. The formation of the chloride ion (Cl⁻) through gaining one electron is a classic example of ionic bond formation.
2. Covalent Bonds
Covalent bonds form when atoms share electrons to achieve a stable octet. This is common among nonmetals. Atoms share valence electrons to fill their outermost shells. For instance, two chlorine atoms can share one electron pair to form a Cl₂ molecule, where each chlorine atom effectively has eight valence electrons (octet).
3. Metallic Bonds
Metallic bonds are formed between metal atoms. Valence electrons are delocalized and move freely among the metal atoms, creating a "sea" of electrons. This results in the characteristic properties of metals, such as high electrical and thermal conductivity and malleability.
Applications and Further Exploration
Understanding the number of valence electrons is crucial in various fields:
- Chemistry: Predicting chemical reactivity, formulating chemical equations, understanding reaction mechanisms.
- Materials Science: Designing new materials with specific properties based on the bonding characteristics of elements.
- Biochemistry: Understanding the interactions of atoms within biological molecules like proteins and DNA.
- Physics: Studying the electronic structure of materials and their behavior in various environments.
Further explorations could include investigating the periodic trends in valence electrons, exploring the exceptions to the octet rule, and delving into more complex bonding scenarios involving coordination compounds and other advanced chemical concepts.
Conclusion
In summary, a neutral chlorine atom has seven valence electrons. However, when it gains one electron to form the chloride ion (Cl⁻), it acquires a stable octet, resulting in eight valence electrons. This understanding of valence electrons and their role in chemical bonding is fundamental to comprehending the vast world of chemistry and its applications. The seemingly simple question of how many valence electrons chloride possesses opens a door to a complex and fascinating world of atomic structure and chemical interactions. The ability to accurately determine valence electrons allows scientists to predict and understand chemical reactions, design new materials, and explore the fundamental building blocks of matter.
Latest Posts
Latest Posts
-
3 1 4 As Improper Fraction
May 09, 2025
-
What Organelles Involved In Protein Synthesis
May 09, 2025
-
How Many Feet Is 25 Centimeters
May 09, 2025
-
Size Chart In Cm And Inches
May 09, 2025
-
How Heat Is Different From Temperature
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does Chloride Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.