How Many Unpaired Electrons Does Phosphorus Have
Juapaving
Mar 14, 2025 · 5 min read
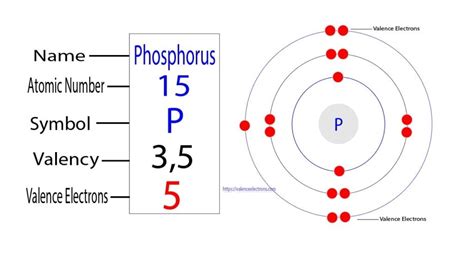
Table of Contents
How Many Unpaired Electrons Does Phosphorus Have? A Deep Dive into Atomic Structure and Electron Configuration
Phosphorus, a nonmetal element crucial to life and numerous industrial applications, presents an interesting case study in atomic structure and electron configuration. Understanding the number of unpaired electrons it possesses requires exploring its electron shell arrangement, its position on the periodic table, and the principles of Hund's rule. This article will delve into these aspects, providing a comprehensive answer to the question: how many unpaired electrons does phosphorus have?
Understanding Electron Configuration and the Periodic Table
Before we determine the number of unpaired electrons in phosphorus, let's establish a fundamental understanding of electron configuration and how it relates to the periodic table. The periodic table organizes elements based on their atomic number (number of protons) and electron configuration. The electron configuration describes how electrons are distributed within the various energy levels (shells) and sublevels (orbitals) of an atom.
Each electron shell can hold a specific maximum number of electrons. The first shell (n=1) can accommodate a maximum of two electrons, the second shell (n=2) up to eight, the third shell (n=3) up to 18, and so on. Within each shell, electrons occupy sublevels denoted by s, p, d, and f orbitals. Each s orbital holds a maximum of two electrons, each p orbital holds a maximum of six electrons (three p orbitals), each d orbital holds a maximum of ten electrons (five d orbitals), and each f orbital holds a maximum of fourteen electrons (seven f orbitals).
The filling of these orbitals follows specific rules, including the Aufbau principle (electrons fill the lowest energy levels first), the Pauli exclusion principle (no two electrons can have the same four quantum numbers), and Hund's rule (electrons will individually occupy each orbital within a subshell before doubling up).
Phosphorus's Position and Properties
Phosphorus (P) is located in Group 15 (also known as Group VA or the pnictogens) and Period 3 of the periodic table. Its atomic number is 15, meaning it has 15 protons and 15 electrons in a neutral atom. This group placement gives us crucial clues about its electron configuration and chemical behavior. Group 15 elements are characterized by having five valence electrons – electrons in the outermost shell that participate in chemical bonding.
Determining Phosphorus's Electron Configuration
To determine the number of unpaired electrons, we need to write out phosphorus's electron configuration. Following the Aufbau principle, the electron configuration of phosphorus is:
1s² 2s² 2p⁶ 3s² 3p³
Let's break this down:
- 1s²: Two electrons fill the 1s orbital.
- 2s²: Two electrons fill the 2s orbital.
- 2p⁶: Six electrons fill the three 2p orbitals (2px, 2py, 2pz).
- 3s²: Two electrons fill the 3s orbital.
- 3p³: Three electrons fill the three 3p orbitals.
Applying Hund's Rule to Determine Unpaired Electrons
This is where Hund's rule becomes critical. Hund's rule states that electrons will singly occupy each orbital within a subshell before pairing up. This minimizes electron-electron repulsion and results in a lower energy state for the atom.
Looking at the 3p³ configuration, we have three electrons to distribute across three 3p orbitals. Following Hund's rule, each of these three electrons will occupy a separate 3p orbital before any pairing occurs. Therefore:
- 3px¹: One electron occupies the 3px orbital.
- 3py¹: One electron occupies the 3py orbital.
- 3pz¹: One electron occupies the 3pz orbital.
The Answer: Phosphorus has Three Unpaired Electrons
Based on the electron configuration and the application of Hund's rule, phosphorus has three unpaired electrons. These unpaired electrons are responsible for phosphorus's reactivity and ability to form three covalent bonds. This explains why phosphorus commonly exhibits a +3 oxidation state in many of its compounds.
The Significance of Unpaired Electrons in Phosphorus's Chemistry
The presence of three unpaired electrons significantly influences phosphorus's chemical behavior and properties:
- Reactivity: The unpaired electrons make phosphorus relatively reactive, readily participating in chemical reactions to achieve a more stable electron configuration (usually by gaining or sharing electrons to complete its octet).
- Bonding: Phosphorus can form three single covalent bonds by sharing its three unpaired electrons with other atoms.
- Paramagnetism: Substances with unpaired electrons are paramagnetic, meaning they are weakly attracted to magnetic fields. Phosphorus exhibits this property due to its three unpaired electrons.
- Oxidation States: The ability of phosphorus to gain or lose electrons leads to various oxidation states, with +3 and +5 being common.
Phosphorus Allotropes and Unpaired Electrons
It's important to note that the number of unpaired electrons is primarily discussed in the context of isolated phosphorus atoms. However, phosphorus exists in several allotropic forms (different structural modifications of the same element), each with different bonding arrangements and potentially altered electron pairing. While the elemental phosphorus atom itself has three unpaired electrons, the situation becomes more complex in its various allotropes like white phosphorus (P₄), red phosphorus, and black phosphorus. The bonding in these allotropes leads to different electron distributions and might involve some electron pairing depending on the specific structure.
Further Applications and Considerations
Understanding the number of unpaired electrons in phosphorus is essential in various fields:
- Inorganic Chemistry: Predicting the reactivity and bonding patterns of phosphorus compounds in fertilizers, flame retardants, and other industrial applications.
- Organic Chemistry: Understanding the role of phosphorus in biological molecules like DNA and RNA, as well as in organophosphorus compounds.
- Material Science: Designing new materials with specific magnetic or electronic properties based on phosphorus's unique electronic structure.
Conclusion
In conclusion, the question of how many unpaired electrons phosphorus has is answered definitively by its electron configuration and Hund's rule. A neutral phosphorus atom possesses three unpaired electrons located in its 3p orbitals. This fundamental aspect of its electronic structure dictates its reactivity, bonding capabilities, and overall chemical behavior. The understanding of unpaired electrons is not just an academic exercise; it underpins our comprehension of phosphorus's role in various scientific disciplines and its diverse applications in numerous industrial and biological contexts. Further exploration into the intricacies of phosphorus allotropes and their specific bonding structures provides additional layers of complexity to this fascinating element.
Latest Posts
Latest Posts
-
Least Common Multiple Of 20 And 24
Mar 14, 2025
-
What Is A Non Zero Constant
Mar 14, 2025
-
How Many Black Cards In A Deck Of 52
Mar 14, 2025
-
Are The Diagonals In A Parallelogram Perpendicular
Mar 14, 2025
-
What Is 2 10 As A Percent
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Does Phosphorus Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
