How Many Protons Neutrons And Electrons Are In Sodium
Juapaving
Mar 14, 2025 · 5 min read
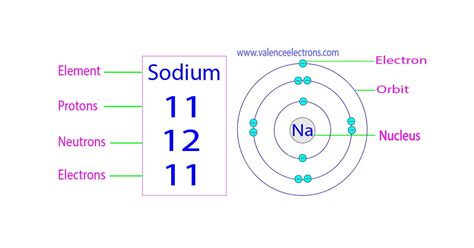
Table of Contents
How Many Protons, Neutrons, and Electrons are in Sodium? A Deep Dive into Atomic Structure
Sodium, a vital element for human life and a common ingredient in various applications, presents a fascinating case study in atomic structure. Understanding the number of protons, neutrons, and electrons within a sodium atom is fundamental to comprehending its chemical properties and behavior. This comprehensive guide delves into the specifics of sodium's atomic composition, exploring related concepts and addressing common misconceptions.
Understanding Atomic Structure: The Building Blocks of Matter
Before diving into the specifics of sodium, let's establish a foundational understanding of atomic structure. An atom is the smallest unit of an element that retains its chemical properties. It consists of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's atomic number and its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. Neutrons contribute to an atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons generally equals the number of protons in a neutral atom.
The arrangement of these subatomic particles dictates an atom's chemical behavior and its interactions with other atoms.
Sodium's Atomic Identity: Unveiling the Numbers
Sodium (Na), an alkali metal, occupies the 11th position on the periodic table. This positioning is crucial because:
-
Atomic Number: The atomic number of an element signifies the number of protons in its nucleus. Sodium's atomic number is 11; therefore, a sodium atom possesses 11 protons.
-
Electrons in a Neutral Atom: In a neutral atom, the number of electrons is equal to the number of protons. Consequently, a neutral sodium atom contains 11 electrons.
-
Neutrons: The Variable Factor: The number of neutrons can vary within the same element, giving rise to isotopes. The most common isotope of sodium, ²³Na, has 12 neutrons. However, other isotopes of sodium exist with varying neutron counts.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties significantly. While the chemical behavior remains largely consistent, variations in mass can lead to subtle differences in physical properties.
Sodium has several isotopes, including:
- ²²Na: This isotope has 11 protons and 11 neutrons.
- ²³Na: This is the most abundant isotope, with 11 protons and 12 neutrons.
- ²⁴Na: This isotope contains 11 protons and 13 neutrons.
While the number of protons and electrons remains constant for all sodium isotopes, the neutron number changes. This leads to variations in atomic mass, typically represented as a superscript before the element symbol (e.g., ²³Na).
Electron Configuration: Orbiting the Nucleus
Understanding electron configuration helps explain sodium's reactivity. Electrons occupy specific energy levels or shells around the nucleus. These shells have a limited capacity for electrons. Sodium's electron configuration is 2, 8, 1. This means:
- First shell (K shell): 2 electrons
- Second shell (L shell): 8 electrons
- Third shell (M shell): 1 electron
This single electron in the outermost shell makes sodium highly reactive. It readily loses this electron to achieve a stable octet (eight electrons) in its outer shell, forming a +1 ion (Na⁺). This tendency to lose an electron explains sodium's strong reducing properties.
Sodium's Role in Biology and Chemistry
The unique atomic structure of sodium directly influences its crucial roles in various biological and chemical processes.
Biological Significance:
Sodium ions (Na⁺) are essential for numerous biological functions, including:
- Nerve impulse transmission: The movement of sodium ions across nerve cell membranes is critical for generating and transmitting nerve impulses.
- Muscle contraction: Sodium ions play a vital role in muscle contraction and relaxation.
- Fluid balance: Sodium helps regulate fluid balance in the body.
- Nutrient absorption: Sodium aids in the absorption of nutrients in the digestive system.
Chemical Applications:
Sodium's reactivity makes it a valuable component in various chemical applications:
- Sodium lamps: Sodium vapor lamps produce a characteristic yellow light, utilized for street lighting and other applications.
- Sodium hydroxide (NaOH) production: Sodium is a precursor in the production of sodium hydroxide, a crucial industrial chemical used in various processes.
- Organic synthesis: Sodium compounds are used extensively as reagents in organic synthesis.
- Sodium chloride (NaCl): Commonly known as table salt, it's crucial in food preservation and flavor enhancement.
Common Misconceptions about Atomic Structure
Several misconceptions surround atomic structure, particularly concerning isotopes and ions:
- Isotopes are different elements: Isotopes are not different elements. They are variations of the same element, differing only in their neutron count. They maintain the same atomic number (number of protons) and thus the same chemical properties.
- Ions have different numbers of protons: Ions have a different number of electrons compared to their neutral counterparts, leading to a net positive (cation) or negative (anion) charge. The number of protons, however, remains the same; this determines the element's identity. A sodium ion (Na⁺) still has 11 protons, but it has only 10 electrons.
- Atomic mass is always a whole number: The atomic mass listed on the periodic table is a weighted average of the masses of all naturally occurring isotopes of an element. It’s not always a whole number because it reflects the abundance of each isotope.
Conclusion: A Deeper Understanding of Sodium
This detailed exploration demonstrates that the seemingly simple question of "How many protons, neutrons, and electrons are in sodium?" opens a gateway to a deeper understanding of atomic structure, isotopic variations, electron configuration, and the element's significant roles in biology and chemistry. Remembering that the number of protons defines the element, while the neutron count creates isotopes, and the electron count dictates the atom's charge and reactivity, provides a strong foundation for understanding the behavior of sodium and other elements. The seemingly simple atom of sodium becomes a fascinating model representing the complexities and interconnectedness within the world of chemistry and biology.
Latest Posts
Latest Posts
-
How Heat Is Different From Temperature
May 09, 2025
-
Where Are Calcium Ions Stored In The Muscle Cell
May 09, 2025
-
The Neutral Particle In The Nucleus Of An Atom
May 09, 2025
-
Label The Structures Of The Vertebrae
May 09, 2025
-
4 6 Rounded To The Nearest Tenth
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Protons Neutrons And Electrons Are In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.