How Many Oxygen Molecules Can Hemoglobin Carry
Juapaving
Mar 28, 2025 · 5 min read
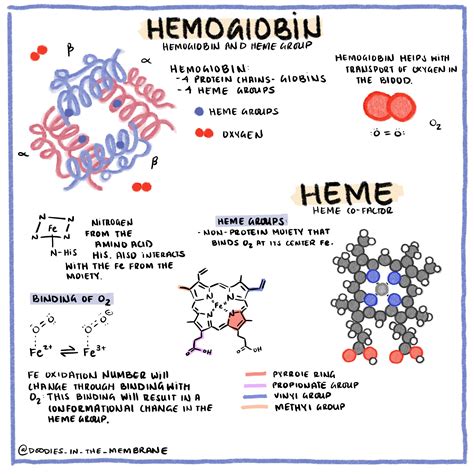
Table of Contents
How Many Oxygen Molecules Can Hemoglobin Carry? A Deep Dive into Oxygen Transport
Oxygen, the life-sustaining gas, is transported throughout our bodies via the blood, specifically bound to hemoglobin, a remarkable protein found within red blood cells. Understanding the precise capacity of hemoglobin to carry oxygen is crucial for comprehending respiratory physiology and various health conditions affecting oxygen transport. This article delves deep into the intricacies of hemoglobin's oxygen-carrying capacity, exploring its structure, function, and the factors that influence its efficiency.
The Hemoglobin Molecule: A Marvel of Molecular Engineering
Hemoglobin, a tetrameric protein, is the primary oxygen transporter in vertebrates. Its structure is exquisitely designed to facilitate efficient oxygen binding and release. Each hemoglobin molecule comprises four subunits, each containing a heme group. These subunits are remarkably similar to myoglobin, the oxygen-storage protein found in muscle tissue.
Hemoglobin's Subunits and Heme Groups
The four subunits in adult hemoglobin (HbA) consist of two alpha (α) and two beta (β) globin chains. Each globin chain cradles a heme group, a porphyrin ring complex containing a ferrous ion (Fe²⁺). This iron ion is the crucial component responsible for binding oxygen. The precise arrangement of these subunits and the heme groups allows for cooperative binding, a phenomenon that significantly enhances oxygen uptake and release.
The Cooperative Binding of Oxygen: A Key to Efficiency
The binding of the first oxygen molecule to a heme group induces a conformational change in the hemoglobin molecule. This change increases the affinity of the remaining heme groups for oxygen, leading to a sigmoidal oxygen-hemoglobin dissociation curve. This cooperative binding means that hemoglobin's affinity for oxygen increases as more oxygen molecules bind, making oxygen uptake in the lungs highly efficient. Conversely, as oxygen partial pressure decreases in the tissues, the release of oxygen is similarly facilitated by the cooperative effect, ensuring adequate oxygen delivery to metabolically active cells.
Calculating Hemoglobin's Oxygen-Carrying Capacity
So, how many oxygen molecules can a single hemoglobin molecule carry? Since each of the four heme groups can bind one oxygen molecule, a single hemoglobin molecule can carry a maximum of four oxygen molecules. This seemingly simple answer, however, requires a nuanced understanding of several factors that can influence the actual number of oxygen molecules bound under various physiological conditions.
Factors Affecting Oxygen Binding: Beyond the Basics
Several factors significantly impact the efficiency of oxygen binding to hemoglobin:
-
Partial Pressure of Oxygen (pO₂): The partial pressure of oxygen in the surrounding environment directly influences the saturation of hemoglobin. Higher pO₂ in the lungs leads to greater hemoglobin saturation, while lower pO₂ in the tissues promotes oxygen release. The oxygen-hemoglobin dissociation curve visually represents this relationship.
-
pH (Bohr Effect): A decrease in pH (increased acidity) reduces hemoglobin's affinity for oxygen. This effect, known as the Bohr effect, is crucial for efficient oxygen delivery to tissues with high metabolic activity, where CO₂ production leads to lower pH. The lower pH causes hemoglobin to release more oxygen.
-
Temperature: Increased temperature also decreases hemoglobin's affinity for oxygen. This is particularly relevant during exercise when body temperature rises. The increased temperature enhances oxygen unloading in active muscles.
-
2,3-Bisphosphoglycerate (2,3-BPG): This molecule, present in red blood cells, binds to hemoglobin and reduces its affinity for oxygen. Increased levels of 2,3-BPG, often seen at high altitudes or in certain disease states, can shift the oxygen-hemoglobin dissociation curve to the right, facilitating oxygen release in the tissues.
-
Carbon Monoxide (CO): Carbon monoxide binds to hemoglobin with much higher affinity than oxygen. This competitive binding reduces the amount of oxygen that can be transported, leading to carbon monoxide poisoning, a potentially life-threatening condition.
-
Carbon Dioxide (CO₂): Besides influencing pH, carbon dioxide also directly binds to hemoglobin, forming carbaminohemoglobin. This reduces the amount of oxygen that can bind, although the effect is less significant than the Bohr effect.
Clinical Implications and Disease States
Understanding hemoglobin's oxygen-carrying capacity is paramount in diagnosing and managing various clinical conditions:
-
Anemia: Anemia, characterized by reduced red blood cell count or hemoglobin levels, directly impacts the oxygen-carrying capacity of the blood. This leads to reduced oxygen delivery to tissues, resulting in fatigue, weakness, and shortness of breath. Different types of anemia affect hemoglobin's ability to bind oxygen in distinct ways.
-
High-Altitude Adaptation: At high altitudes, the partial pressure of oxygen is significantly lower. Individuals acclimatizing to high altitudes often experience increased levels of 2,3-BPG, which shifts the oxygen-hemoglobin dissociation curve to the right, facilitating oxygen unloading in the tissues despite the lower oxygen availability.
-
Sickle Cell Anemia: In sickle cell anemia, a mutation in the beta-globin chain alters hemoglobin's structure, resulting in sickle-shaped red blood cells. These abnormal cells have impaired oxygen transport and can lead to vascular blockages and other serious complications.
-
Thalassemia: Thalassemia involves reduced or absent production of either alpha or beta globin chains, impacting the structure and function of hemoglobin. This leads to impaired oxygen transport and various clinical manifestations.
-
Carbon Monoxide Poisoning: As previously mentioned, carbon monoxide poisoning severely impairs hemoglobin's oxygen-carrying capacity, leading to life-threatening hypoxia.
Conclusion: The Dynamic Nature of Hemoglobin's Function
While a single hemoglobin molecule can carry four oxygen molecules, the actual number bound under physiological conditions is a dynamic variable influenced by numerous factors. Understanding the complex interplay of these factors is crucial for comprehending oxygen transport in health and disease. The intricate structure and cooperative binding properties of hemoglobin highlight the remarkable efficiency of our body's oxygen transport system, a testament to the elegance of biological design. Further research continues to unravel the complexities of hemoglobin's function and its crucial role in maintaining life. This knowledge forms the basis for developing effective treatments and management strategies for various respiratory and hematological disorders. The ongoing study of hemoglobin and its interaction with oxygen remains a vital area of medical and biological research, constantly refining our understanding of this fundamental life process.
Latest Posts
Latest Posts
-
350 Square Meters In Square Feet
Mar 31, 2025
-
Explain How Software Is Distinct From Hardware
Mar 31, 2025
-
Greater Than Less Than Decimals Calculator
Mar 31, 2025
-
Father Of The Constitution Of India
Mar 31, 2025
-
Whats The Roman Numeral For 35
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about How Many Oxygen Molecules Can Hemoglobin Carry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
