How Many Neutrons Are In Na
Juapaving
Mar 24, 2025 · 5 min read
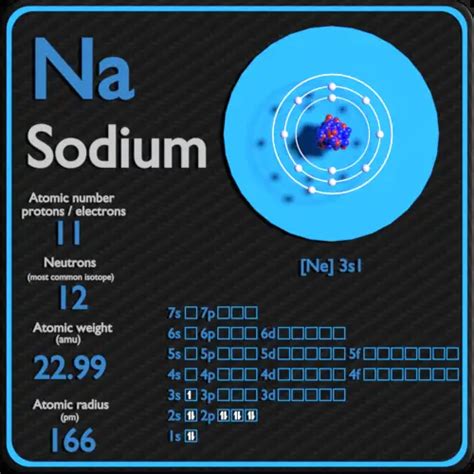
Table of Contents
How Many Neutrons Are in Na? Understanding Isotopes and Atomic Structure
Sodium (Na), a crucial element for life, presents a fascinating case study in atomic structure, particularly concerning the number of neutrons it possesses. Unlike the fixed number of protons defining an element, the number of neutrons can vary, leading to the concept of isotopes. This article will delve deep into understanding sodium's atomic structure, explore its various isotopes, and clarify how to determine the number of neutrons present in a specific sodium atom.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle the neutron count in sodium, let's refresh our understanding of atomic structure. An atom consists of three fundamental subatomic particles:
-
Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the atomic number of an element and determines its identity. Sodium, for example, always has 11 protons.
-
Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. Unlike protons, the number of neutrons can vary within an element, leading to isotopes.
-
Electrons: Negatively charged particles orbiting the nucleus in electron shells. The number of electrons typically equals the number of protons in a neutral atom, maintaining electrical neutrality.
Isotopes: The Key to Variable Neutron Numbers
The term isotope refers to atoms of the same element (same number of protons) that have different numbers of neutrons. This difference in neutron count affects the atom's mass but not its chemical properties. Isotopes are often represented using the element's symbol with the mass number as a superscript. The mass number is the sum of protons and neutrons.
For example, ²³Na represents a sodium isotope with a mass number of 23. Since sodium always has 11 protons, this isotope has 23 - 11 = 12 neutrons.
Determining the Number of Neutrons in Sodium (Na)
To determine the number of neutrons in a sodium atom, we need two pieces of information:
-
Atomic Number: The atomic number of sodium (Na) is always 11. This indicates the presence of 11 protons.
-
Mass Number: This is the total number of protons and neutrons in the nucleus. The mass number is usually indicated as a superscript to the left of the element's symbol (e.g., ²³Na). If the mass number isn't provided, you'll need to consult a periodic table which often lists the average atomic mass. This average reflects the natural abundance of different sodium isotopes.
Formula:
The number of neutrons can be calculated using the following simple formula:
Number of Neutrons = Mass Number - Atomic Number
Example:
Let's consider the isotope ²³Na.
- Mass Number = 23
- Atomic Number = 11
Number of Neutrons = 23 - 11 = 12 neutrons
Therefore, the isotope ²³Na has 12 neutrons.
The Abundance of Sodium Isotopes: A Closer Look
Sodium has several known isotopes, but only one is stable and naturally abundant: ²³Na. This isotope makes up nearly 100% of naturally occurring sodium. Other isotopes, such as ²²Na, are radioactive and have much shorter half-lives. These radioactive isotopes find applications in various fields like medical imaging and industrial tracing.
While the vast majority of sodium atoms encountered will have 12 neutrons (²³Na), it's crucial to remember that other isotopes exist, albeit in negligible quantities in nature.
Applications of Sodium and its Isotopes
Understanding the atomic structure of sodium, including its neutron count, is crucial due to its widespread applications across numerous fields:
1. Biological Significance:
Sodium plays a vital role in maintaining the body's fluid balance, nerve impulse transmission, and muscle contraction. Its presence and concentration are tightly regulated within biological systems.
2. Industrial Uses:
Sodium is extensively used in various industrial processes, including:
- Sodium lamps: These energy-efficient lamps produce a characteristic yellow light.
- Sodium hydroxide (NaOH) production: NaOH, a strong base, is crucial in various industrial applications, such as soap making and paper production.
- Chemical synthesis: Sodium is a reactant in numerous chemical reactions, acting as a reducing agent or a catalyst.
- Nuclear reactors: Certain sodium isotopes may find use in specific applications within nuclear reactors, although this is highly specialized.
3. Medical Applications:
Radioactive sodium isotopes, such as ²²Na, are employed in:
- Medical imaging: Radioactive tracers, including those containing sodium isotopes, can be used to visualize internal organs and tissues, aiding in disease diagnosis.
- Positron Emission Tomography (PET) scans: These scans utilize radioactive tracers to monitor metabolic processes in the body.
Beyond the Basics: Nuclear Stability and Isotope Decay
The number of neutrons significantly influences an atom's nuclear stability. A stable nucleus has a specific neutron-to-proton ratio. Isotopes with unstable nuclei undergo radioactive decay to achieve greater stability, emitting particles like alpha, beta, or gamma radiation. The decay process transforms the unstable isotope into a different element or a more stable isotope. The half-life of a radioactive isotope refers to the time it takes for half of the initial amount to decay.
Understanding isotope decay is crucial for various fields, including nuclear medicine, environmental monitoring, and geological dating.
Conclusion: The Significance of Neutron Count in Sodium
The number of neutrons in a sodium atom isn't a static value; it varies depending on the isotope. While the most abundant and stable isotope, ²³Na, contains 12 neutrons, other, less common, isotopes exist, each possessing a different neutron count. This understanding is crucial not just for theoretical studies in nuclear physics and chemistry but also for the numerous practical applications of sodium and its isotopes in various fields, including biology, medicine, and industry. Remember that determining the number of neutrons always requires knowing both the atomic number (always 11 for sodium) and the mass number of the specific sodium isotope in question. This knowledge is fundamental to understanding the properties and behavior of this essential element.
Latest Posts
Latest Posts
-
What Can 81 Be Divided By
Mar 29, 2025
-
A Number Is Divisible By 6 If
Mar 29, 2025
-
What Stores Water In Plant Cells
Mar 29, 2025
-
Exercise On Transitive And Intransitive Verbs
Mar 29, 2025
-
What Ratio Is Equivalent To 4 5
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about How Many Neutrons Are In Na . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
