How Many Lone Pairs Does Nh3 Have
Juapaving
May 13, 2025 · 5 min read
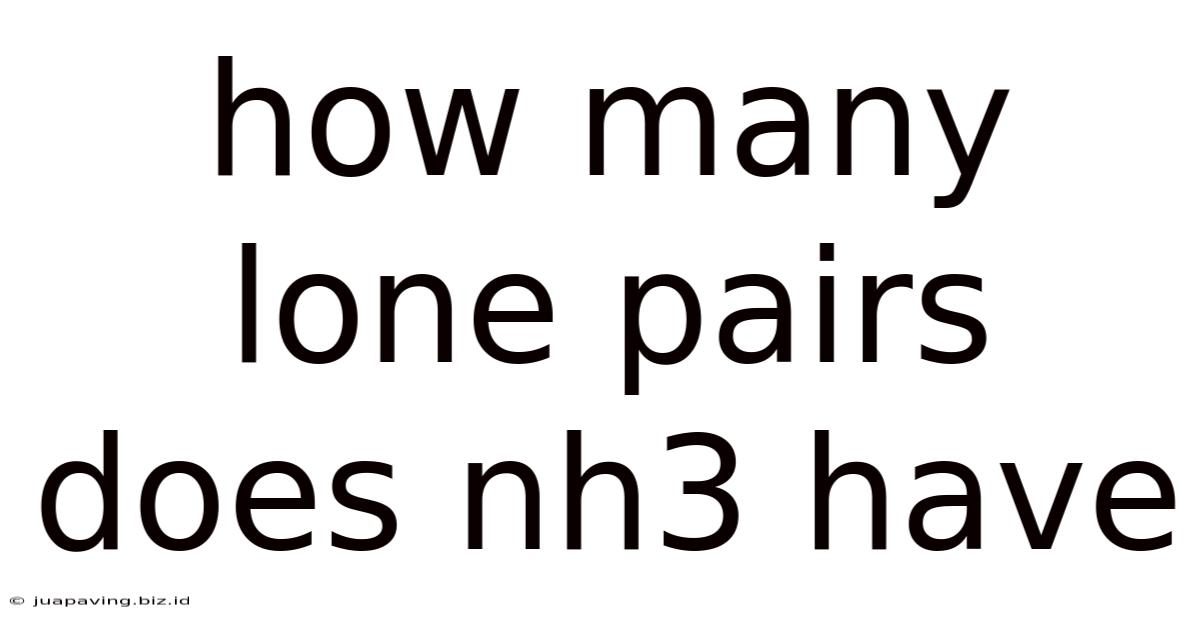
Table of Contents
How Many Lone Pairs Does NH₃ Have? A Deep Dive into Ammonia's Structure and Bonding
Ammonia (NH₃), a ubiquitous compound in nature and industry, holds a fascinating place in chemistry due to its simple yet intriguing structure. Understanding its bonding, particularly the number of lone pairs, is crucial to grasping its reactivity and properties. This article delves into the intricacies of ammonia's molecular geometry, exploring its lone pairs and their impact on its behavior. We'll also touch upon related concepts like hybridization and VSEPR theory, providing a comprehensive understanding of this fundamental chemical species.
Understanding Ammonia's Molecular Structure
Before we tackle the number of lone pairs, let's establish a foundational understanding of ammonia's structure. Ammonia is a covalent compound, meaning its atoms share electrons to achieve a stable electron configuration. Nitrogen (N), with five valence electrons, forms three single covalent bonds with three hydrogen (H) atoms, each contributing one valence electron.
The Role of Valence Electrons
Each covalent bond involves the sharing of two electrons, one from each participating atom. In NH₃, nitrogen forms three such bonds, utilizing six of its five valence electrons. This leaves one electron pair unbonded. This is what's crucial for determining the number of lone pairs in NH₃.
Determining Lone Pairs: A Step-by-Step Approach
- Valence Electrons of Nitrogen: Nitrogen possesses five valence electrons.
- Electrons Used in Bonding: Three electrons are used to form three single covalent bonds with three hydrogen atoms.
- Remaining Electrons: This leaves 5 - 3 = 2 electrons.
- Lone Pairs: Since two electrons constitute a lone pair, ammonia (NH₃) has one lone pair of electrons.
The VSEPR Theory and Ammonia's Geometry
The Valence Shell Electron Pair Repulsion (VSEPR) theory is a powerful tool for predicting the three-dimensional arrangement of atoms in a molecule. It postulates that electron pairs, both bonding and non-bonding (lone pairs), repel each other and arrange themselves to minimize this repulsion.
Applying VSEPR to Ammonia
In NH₃, we have four electron regions around the central nitrogen atom: three bonding pairs and one lone pair. According to VSEPR, these electron regions arrange themselves in a tetrahedral geometry to minimize repulsion. However, the molecular geometry (considering only the positions of the atoms) is trigonal pyramidal. The lone pair occupies more space than the bonding pairs, pushing the hydrogen atoms slightly closer together. This results in bond angles of approximately 107°, slightly less than the ideal tetrahedral angle of 109.5°.
Hybridization: Understanding the Orbitals
The concept of hybridization helps explain the bonding in ammonia. Nitrogen's ground state electronic configuration is 1s²2s²2p³. However, to form three equivalent bonds with hydrogen, nitrogen undergoes sp³ hybridization.
The sp³ Hybridization Process
In sp³ hybridization, one 2s electron and three 2p electrons combine to form four sp³ hybrid orbitals. These four orbitals are identical in energy and shape, and they arrange themselves tetrahedrally. Three of these hybrid orbitals overlap with the 1s orbitals of the hydrogen atoms to form the three N-H sigma bonds. The remaining sp³ hybrid orbital contains the lone pair of electrons.
The Significance of the Lone Pair in Ammonia's Properties
The lone pair of electrons in ammonia plays a critical role in determining its physical and chemical properties. Its presence contributes significantly to:
1. Polarity and Solubility
The lone pair contributes to the overall polarity of the ammonia molecule. Nitrogen is more electronegative than hydrogen, causing the N-H bonds to be polar. The asymmetrical arrangement of the lone pair and the bonding pairs further enhances the molecule's dipole moment, resulting in a polar molecule. This makes ammonia highly soluble in polar solvents like water, due to hydrogen bonding interactions.
2. Hydrogen Bonding
Ammonia's lone pair is crucial for its ability to participate in hydrogen bonding. The lone pair on nitrogen can accept a proton (H⁺) from another molecule, forming a hydrogen bond. This strong intermolecular force is responsible for ammonia's relatively high boiling point compared to other hydrides of similar molecular weight.
3. Basicity and Reactivity
The lone pair makes ammonia a Lewis base. It readily donates the electron pair to electron-deficient species, acting as a nucleophile. This is the basis for its weak basicity in aqueous solutions, where it can accept a proton from water to form ammonium ions (NH₄⁺). This basicity is vital in many chemical reactions, including the synthesis of various nitrogen-containing compounds.
Ammonia in Everyday Life and Industry
The properties stemming from its lone pair make ammonia a versatile compound with broad applications:
- Fertilizers: Ammonia is a crucial component in the production of fertilizers, providing nitrogen, an essential nutrient for plant growth. The Haber-Bosch process, which synthesizes ammonia from nitrogen and hydrogen, is a cornerstone of modern agriculture.
- Cleaning Products: Ammonia is utilized in numerous household cleaning agents due to its ability to dissolve grease and other grime.
- Refrigeration: Its high heat of vaporization makes it an effective refrigerant.
- Pharmaceuticals: It's an intermediate in the synthesis of numerous pharmaceuticals and other fine chemicals.
Conclusion: A Lone Pair with Significant Impact
The seemingly simple answer – ammonia possesses one lone pair of electrons – belies the significant impact this lone pair has on its overall properties and its myriad applications. From its trigonal pyramidal geometry and polarity to its hydrogen bonding capabilities and Lewis basicity, the lone pair drives much of ammonia's chemical behavior. This comprehensive understanding of ammonia's structure and bonding is not merely an academic exercise; it's fundamental to appreciating its pivotal role in various sectors, impacting our daily lives in numerous ways. Further exploration of related compounds and their bonding characteristics provides a deeper understanding of fundamental chemical principles.
Further Exploration
For a deeper understanding, consider investigating these related topics:
- Advanced VSEPR Theory: Explore exceptions to VSEPR rules and how they apply to more complex molecules.
- Molecular Orbital Theory: Gain a more sophisticated understanding of bonding using molecular orbital diagrams.
- Organic Chemistry of Amines: Investigate how the lone pair on nitrogen affects the reactivity of amines (organic compounds containing nitrogen).
- Coordination Chemistry: Explore how ammonia acts as a ligand, donating its lone pair to metal ions.
By exploring these areas, you can develop a more nuanced understanding of the crucial role of lone pairs in chemical bonding and molecular properties. The simple molecule of ammonia serves as a perfect case study to illustrate these fundamental principles.
Latest Posts
Latest Posts
-
What Are The Four Factors That Affect Climate
May 13, 2025
-
The Difference Between Heat And Temperature
May 13, 2025
-
How Many Orbitals In Third Shell
May 13, 2025
-
What Is The Number In Scientific Notation 0 0765
May 13, 2025
-
Loyalty And Devotion To A Nation
May 13, 2025
Related Post
Thank you for visiting our website which covers about How Many Lone Pairs Does Nh3 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.