How Many Electrons Protons And Neutrons Does Sodium Have
Juapaving
Mar 13, 2025 · 6 min read
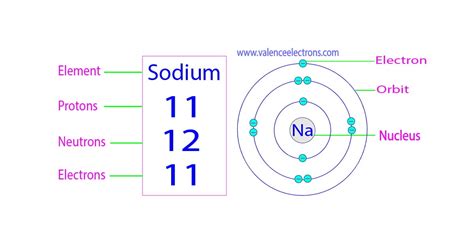
Table of Contents
How Many Electrons, Protons, and Neutrons Does Sodium Have? A Deep Dive into Atomic Structure
Sodium, a ubiquitous element crucial for life and various industrial processes, presents a fascinating case study in atomic structure. Understanding its composition of electrons, protons, and neutrons is fundamental to grasping its chemical behavior and properties. This article delves deep into the atomic makeup of sodium, exploring the concepts of atomic number, mass number, isotopes, and their implications. We'll also touch upon the significance of this elemental composition in broader scientific contexts.
Understanding Atomic Structure: The Basics
Before we delve into the specifics of sodium, let's establish a foundational understanding of atomic structure. An atom, the fundamental building block of matter, consists of three primary subatomic particles:
- Protons: Positively charged particles residing in the atom's nucleus. The number of protons defines the element's identity.
- Neutrons: Neutrally charged particles also found in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. They determine the atom's chemical reactivity.
The arrangement of these particles dictates an atom's properties and how it interacts with other atoms. Crucially, in a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
Sodium's Atomic Number and Mass Number
Sodium's atomic number is 11. This signifies that a neutral sodium atom possesses 11 protons in its nucleus. Since the atom is electrically neutral, it also contains 11 electrons orbiting the nucleus. These electrons are arranged in specific energy levels or shells, with 2 electrons in the first shell, 8 in the second, and 1 in the outermost third shell (valence shell). This single valence electron is responsible for sodium's high reactivity.
The mass number, on the other hand, represents the total number of protons and neutrons in the nucleus. While the atomic number is always constant for a given element, the mass number can vary due to the presence of isotopes. The most common isotope of sodium, Sodium-23 (²³Na), has a mass number of 23. This means it has 11 protons and 12 neutrons (23 - 11 = 12).
Isotopes of Sodium: Variations in Neutron Count
Isotopes are atoms of the same element that have the same number of protons but differ in their number of neutrons. This difference in neutron count leads to variations in the atom's mass. While Sodium-23 is the most prevalent isotope of sodium, other isotopes exist, though they are far less abundant and often radioactive.
Some examples of less common sodium isotopes include:
- Sodium-22 (²²Na): This isotope has 11 protons and 11 neutrons. It's radioactive and decays through positron emission.
- Sodium-24 (²⁴Na): This isotope has 11 protons and 13 neutrons. It's also radioactive and is often used as a tracer in medical and industrial applications.
The existence of isotopes explains why the atomic mass of sodium listed on the periodic table is approximately 22.99 atomic mass units (amu) and not exactly 23 amu. This average atomic mass reflects the weighted average of the masses of all naturally occurring isotopes of sodium, considering their relative abundances.
Electron Configuration and Chemical Reactivity
The arrangement of electrons in sodium's electron shells plays a pivotal role in its chemical behavior. As mentioned, sodium has 11 electrons, distributed as 2, 8, 1. This single electron in the outermost shell (valence shell) makes sodium highly reactive. It readily loses this electron to achieve a stable octet configuration, similar to the noble gas neon. This electron loss leads to the formation of a positively charged sodium ion (Na⁺). This tendency to readily lose an electron is characteristic of alkali metals, the group to which sodium belongs.
This strong tendency to lose an electron explains sodium's reactivity with water, oxygen, and other elements. The reaction with water is particularly vigorous, producing hydrogen gas and sodium hydroxide, a strongly alkaline solution. This reactivity underscores the importance of storing sodium under inert conditions to prevent dangerous reactions.
Sodium's Importance in Biology and Industry
Sodium's unique atomic structure and chemical properties have far-reaching implications in various fields:
Biological Significance:
- Electrolyte Balance: Sodium ions (Na⁺) are crucial for maintaining electrolyte balance in the human body. This balance is essential for nerve impulse transmission, muscle contraction, and fluid regulation.
- Nutrient Transport: Sodium is involved in the transport of nutrients across cell membranes.
- Osmotic Pressure: Sodium contributes to maintaining osmotic pressure, which regulates fluid movement between cells and their surroundings.
Industrial Applications:
- Sodium Lamps: Sodium vapor lamps produce a characteristic yellow light, making them efficient and widely used in street lighting.
- Chemical Industry: Sodium is a crucial reactant in various chemical processes, including the production of many important compounds.
- Sodium-Sulfur Batteries: Sodium is used in innovative battery technologies due to its high reactivity and abundance.
Beyond the Basics: Deeper Exploration of Isotopes and Nuclear Physics
The study of sodium isotopes extends beyond simply counting protons and neutrons. Radioactive isotopes like Sodium-22 and Sodium-24 have important applications in various scientific and medical fields:
- Medical Imaging: Radioactive isotopes, when administered in controlled doses, can be used in medical imaging techniques like PET (Positron Emission Tomography) scans to diagnose diseases.
- Tracer Studies: Radioactive isotopes can act as tracers, allowing scientists to track the movement and fate of substances within complex systems.
- Nuclear Medicine: Sodium-24, for instance, is utilized in certain nuclear medicine procedures.
The study of isotopes and their decay mechanisms lies within the realm of nuclear physics, a field dedicated to understanding the structure and behavior of atomic nuclei. The nuclear properties of isotopes, including their half-lives and decay modes, are essential for understanding their applications and potential risks.
Conclusion: The Significance of Sodium's Atomic Composition
Understanding the precise number of electrons, protons, and neutrons in a sodium atom, along with the concept of isotopes, is fundamental to grasping its chemical reactivity, biological role, and industrial applications. The single valence electron determines sodium's high reactivity, leading to its participation in numerous crucial processes, from nerve impulse transmission to various chemical reactions. The existence of isotopes adds further complexity and expands the applications of sodium, particularly in medical and scientific research. Exploring the intricacies of sodium's atomic structure provides a compelling illustration of the fundamental principles of chemistry and physics and their profound impact on our world. The careful study of atomic structure reveals not only the building blocks of matter but also the dynamic interactions that shape the physical and biological world around us.
Latest Posts
Latest Posts
-
What Are The Radioactive Elements In The Periodic Table
May 09, 2025
-
How To Balance C8h18 O2 Co2 H2o
May 09, 2025
-
How Are The Desert And Tundra Similar
May 09, 2025
-
What Is Meant By Saying Charge Is Quantized
May 09, 2025
-
Undifferentiated Diploid Spermatogenic Cells Are Called
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Protons And Neutrons Does Sodium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.