How Many Electrons Protons And Neutrons Are In Sodium
Juapaving
Mar 18, 2025 · 5 min read
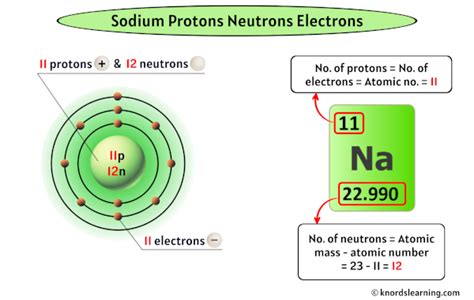
Table of Contents
How Many Electrons, Protons, and Neutrons are in Sodium? A Deep Dive into Atomic Structure
Understanding the composition of atoms is fundamental to chemistry and physics. This article delves into the atomic structure of sodium (Na), specifically exploring the number of electrons, protons, and neutrons it possesses. We'll also explore related concepts like atomic number, mass number, isotopes, and their implications.
Understanding Atomic Structure: The Building Blocks of Matter
Before we delve into the specifics of sodium, let's establish a foundational understanding of atomic structure. Atoms are the fundamental building blocks of all matter. They consist of three primary subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus (center). The number of protons defines the element.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons typically equals the number of protons in a neutral atom.
Sodium (Na): A Closer Look at its Atomic Composition
Sodium, a highly reactive alkali metal, is a crucial element in various biological and industrial processes. Its atomic symbol is Na (from the Latin word natrium), and it's characterized by its unique atomic structure.
Determining the Number of Protons
The atomic number of an element determines the number of protons in its nucleus. Sodium's atomic number is 11. This means every sodium atom possesses 11 protons. This is a defining characteristic; if an atom has 11 protons, it is, by definition, a sodium atom. Changing the number of protons fundamentally changes the element itself.
Determining the Number of Electrons
In a neutral sodium atom, the number of electrons equals the number of protons. Therefore, a neutral sodium atom has 11 electrons. This balanced charge ensures the atom is electrically neutral, with the positive charge of the protons being cancelled out by the negative charge of the electrons. However, sodium readily loses one electron to achieve a stable electron configuration, forming a positively charged ion (Na⁺) with 10 electrons.
Determining the Number of Neutrons: The Role of Isotopes
Determining the number of neutrons is slightly more complex. The mass number of an atom is the sum of its protons and neutrons. Unlike the atomic number which is constant for a given element, the mass number can vary. This variation arises due to isotopes.
Isotopes are atoms of the same element (same number of protons) that have different numbers of neutrons. Sodium has several isotopes, the most common being Sodium-23 (²³Na) and less abundant isotopes like Sodium-22 (²²Na).
-
Sodium-23 (²³Na): This is the most abundant isotope of sodium. Its mass number is 23. Since it has 11 protons (atomic number), it has 23 - 11 = 12 neutrons.
-
Sodium-22 (²²Na): This isotope has a mass number of 22. Therefore, it has 22 - 11 = 11 neutrons.
Isotopes and Their Significance
The existence of isotopes is a significant aspect of understanding the behavior of elements. While isotopes have the same chemical properties (due to the same number of protons and electrons), they can have different physical properties due to differences in mass. For instance, some isotopes are radioactive, meaning they spontaneously decay, emitting radiation. Sodium-22 is a radioactive isotope used in various medical applications, while Sodium-23 is stable.
The abundance of each isotope varies in nature. When we talk about the average atomic mass of an element (like sodium's 22.99 amu on the periodic table), it's a weighted average reflecting the abundance of each isotope.
Applications of Sodium and its Isotopes
Sodium's unique properties make it essential in various applications:
-
Biological Roles: Sodium ions (Na⁺) play a crucial role in maintaining fluid balance, nerve impulse transmission, and muscle contraction in living organisms.
-
Industrial Uses: Sodium is used in the production of various chemicals, including sodium hydroxide (NaOH) and sodium carbonate (Na₂CO₃), both vital in many industries.
-
Sodium Lamps: Sodium vapor lamps produce a bright yellow light, commonly used in street lighting.
-
Nuclear Medicine: Sodium-22, being a radioactive isotope, finds applications in nuclear medicine as a tracer in various diagnostic procedures.
Beyond the Basics: Electron Configuration and Chemical Reactivity
The arrangement of electrons in an atom's shells determines its chemical reactivity. Sodium's electron configuration is 1s²2s²2p⁶3s¹. This means the outermost shell (3s) contains only one electron. This single electron is easily lost to achieve a stable octet (full outermost shell) configuration, resulting in the formation of a Na⁺ ion. This tendency to lose an electron explains sodium's high reactivity.
Further Exploration: Atomic Mass and its Calculation
The atomic mass of an element, as listed on the periodic table, is a weighted average of the masses of its naturally occurring isotopes, taking into account their relative abundances. The calculation involves multiplying the mass of each isotope by its relative abundance (expressed as a decimal fraction) and summing the results. For sodium, this weighted average yields an atomic mass of approximately 22.99 atomic mass units (amu).
Conclusion: A Complete Picture of Sodium's Atomic Structure
In summary, a neutral sodium atom contains:
- 11 protons in its nucleus
- 11 electrons orbiting the nucleus
- 12 neutrons in its nucleus (for the most abundant isotope, ²³Na)
Understanding these numbers and the underlying concepts of atomic number, mass number, and isotopes provides a comprehensive understanding of sodium's atomic structure and its unique chemical and physical properties. This knowledge forms the basis for exploring sodium's diverse roles in various scientific and technological fields. The existence of isotopes, with their varying neutron numbers, adds another layer of complexity and highlights the diverse forms an element can take while retaining its elemental identity. Furthermore, knowledge of electron configuration allows us to predict and explain sodium's high reactivity. This deeper understanding is crucial for anyone studying chemistry, physics, or related disciplines.
Latest Posts
Latest Posts
-
Steam Produces More Severe Burns Than Boiling Water
Mar 18, 2025
-
Is The Number 3 Prime Or Composite
Mar 18, 2025
-
The Type Of Waves That Require Medium To Pass
Mar 18, 2025
-
What Are The Factors Of 79
Mar 18, 2025
-
What Causes Extra Lobe On Placenta
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Protons And Neutrons Are In Sodium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
