How Many Atoms Are In A Fcc Unit Cell
Juapaving
Mar 14, 2025 · 6 min read
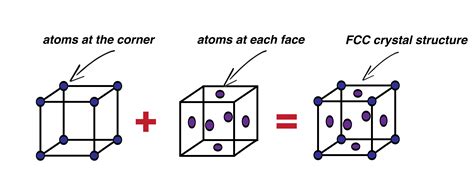
Table of Contents
How Many Atoms Are in an FCC Unit Cell? A Deep Dive into Crystallography
The face-centered cubic (FCC) unit cell is a fundamental building block in understanding the structure of numerous crystalline materials. Understanding its atomic arrangement is crucial in various fields, including materials science, chemistry, and physics. This article delves into the precise calculation of the number of atoms within an FCC unit cell, exploring the underlying concepts and providing a detailed, step-by-step explanation. We will also explore the implications of this atomic arrangement on material properties.
Understanding the FCC Unit Cell Structure
Before determining the atom count, let's visualize the FCC structure. Imagine a cube representing the unit cell. In an FCC arrangement, atoms are located at each of the eight corners of this cube and at the center of each of the six faces. This arrangement leads to a highly efficient packing of atoms, resulting in a high density. This efficient packing contributes significantly to the properties of materials exhibiting this crystal structure. Examples include metals like copper, aluminum, and gold, which are known for their ductility and malleability – properties directly related to their atomic arrangement.
Corner Atoms and Their Contribution
Each of the eight corner atoms is shared equally among eight adjacent unit cells. Therefore, each corner atom contributes only 1/8 of an atom to a single unit cell. This fractional contribution is a key concept in understanding unit cell calculations. We don't simply count eight atoms; instead, we consider the portion of each atom that resides within the unit cell we are analyzing.
Face-Centered Atoms and Their Contribution
The six face-centered atoms present a different scenario. Each face-centered atom is shared equally between two adjacent unit cells. Consequently, each face-centered atom contributes 1/2 of an atom to a single unit cell.
Calculating the Total Number of Atoms
Now, let's combine these contributions to determine the total number of atoms within a single FCC unit cell:
- Corner atoms: 8 corner atoms × (1/8 atom/corner atom) = 1 atom
- Face-centered atoms: 6 face-centered atoms × (1/2 atom/face-centered atom) = 3 atoms
Total atoms per FCC unit cell: 1 atom + 3 atoms = 4 atoms
Therefore, a single FCC unit cell contains a total of four atoms. This seemingly simple calculation is fundamental to understanding the properties and behavior of materials with an FCC crystal structure.
Implications of the FCC Structure and Atomic Arrangement
The FCC structure, with its four atoms per unit cell, leads to several significant material properties:
High Atomic Packing Factor (APF)
The FCC arrangement boasts a high atomic packing factor (APF), which is a measure of how efficiently atoms are packed within the unit cell. For an FCC structure, the APF is approximately 74%, meaning that 74% of the unit cell's volume is occupied by atoms. This high APF contributes to the high density observed in many FCC metals. Compare this to a simple cubic structure, which has a significantly lower APF. The high density is directly correlated with properties like strength and resistance to compression.
Ductility and Malleability
The close-packed nature of the FCC structure facilitates the ability of these materials to deform under stress without fracturing. This is because the atoms can easily slide past each other along densely packed planes, a mechanism known as slip. This characteristic is responsible for the ductility (ability to be drawn into wires) and malleability (ability to be hammered into sheets) observed in many FCC metals. The ease of atomic rearrangement under stress makes these metals suitable for various applications requiring formability.
Isotropy
Many FCC metals exhibit isotropic properties, meaning their properties are the same in all directions. While some anisotropy might exist at the microscopic level, the macroscopic behavior often demonstrates isotropy. This characteristic is valuable in engineering applications where consistent performance in various orientations is required.
Electrical Conductivity
The close proximity and regular arrangement of atoms in the FCC structure facilitates the free movement of electrons. This contributes to the excellent electrical conductivity observed in many FCC metals, making them valuable in electrical wiring and other conductive applications. The ease of electron flow is a direct consequence of the highly ordered structure.
Thermal Conductivity
Similar to electrical conductivity, the efficient atomic arrangement also leads to high thermal conductivity in FCC metals. Heat is efficiently transferred through the lattice due to the close proximity of atoms and their ability to vibrate and transfer energy. This property is important in various applications involving heat transfer.
Applications of FCC Metals
The properties stemming from the FCC structure make these materials essential in a wide range of applications:
-
Aluminum: Used extensively in transportation (aircraft, automobiles), packaging, and construction due to its lightweight nature and good strength-to-weight ratio. Its corrosion resistance is also a major advantage.
-
Copper: Used extensively in electrical wiring, plumbing, and heat exchangers due to its excellent electrical and thermal conductivity. Its corrosion resistance is important in many applications.
-
Gold: Highly valued for jewelry, electronics (as a conductor and in coating applications), and dentistry because of its malleability, ductility, and corrosion resistance.
-
Nickel: Used in various alloys, such as stainless steel and superalloys, offering strength, corrosion resistance, and high-temperature capabilities.
-
Silver: Used in jewelry, electronics, and photography due to its high electrical and thermal conductivity, corrosion resistance, and reflective properties. Its antimicrobial properties also lead to applications in wound care.
Advanced Considerations and Further Exploration
The simple calculation of four atoms per unit cell provides a foundational understanding. However, more complex crystallographic analysis involves considerations such as:
-
Defects: Real crystals are not perfect; they contain various defects (vacancies, interstitials, dislocations) that affect material properties. These defects alter the precise atomic arrangement and can significantly impact material behavior.
-
Alloying: The addition of other elements (alloying) modifies the crystal structure and properties. Alloying can alter the atomic arrangement, potentially leading to changes in the number of atoms per unit cell or modifications to the overall crystal structure.
-
Temperature Effects: Temperature changes can influence the vibrational motion of atoms, affecting various properties. High temperatures can even lead to phase transitions, altering the crystal structure and atom arrangement.
-
X-ray Diffraction: X-ray diffraction techniques are crucial in determining the crystal structure and confirming the presence of an FCC arrangement. Analyzing diffraction patterns provides detailed information about the atomic arrangement and unit cell dimensions.
Conclusion
The determination of four atoms per FCC unit cell is a cornerstone of materials science. Understanding this simple calculation provides insights into the structure-property relationships of numerous materials with widespread technological applications. Further exploration into more complex considerations offers a deeper appreciation of the intricate relationships between crystal structure, atomic arrangement, and macroscopic material properties. The efficiency of the FCC arrangement, its high APF, and resulting material properties underscore the importance of understanding crystal structures in materials science, engineering, and related fields. This knowledge allows for material selection based on desired properties and facilitates the design of advanced materials with tailored characteristics.
Latest Posts
Latest Posts
-
Is Force Increase On An Inclined Plane
May 09, 2025
-
Interesting Words That Start With V
May 09, 2025
-
Compare And Contrast A Light Microscope And An Electron Microscope
May 09, 2025
-
What Is 6 25 Meters In Feet And Inches
May 09, 2025
-
How Many Centimeters Are In 50 Millimeters
May 09, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In A Fcc Unit Cell . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.