How Do You Change Moles To Grams
Juapaving
Mar 10, 2025 · 5 min read
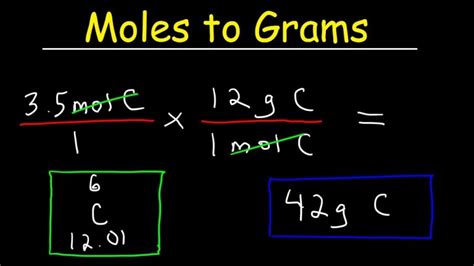
Table of Contents
How Do You Change Moles to Grams? A Comprehensive Guide
Converting moles to grams is a fundamental concept in chemistry, crucial for stoichiometric calculations and understanding chemical reactions. This comprehensive guide will walk you through the process, explaining the underlying principles and providing numerous examples to solidify your understanding. We'll also explore common pitfalls and offer strategies to avoid errors.
Understanding Moles and Grams
Before diving into the conversion process, let's refresh our understanding of moles and grams.
What is a Mole?
A mole (mol) is a unit of measurement in chemistry that represents a specific number of particles, whether atoms, molecules, ions, or formula units. This number, known as Avogadro's number, is approximately 6.022 x 10<sup>23</sup>. Think of it like a dozen – a dozen eggs always means 12 eggs; a mole of any substance always contains 6.022 x 10<sup>23</sup> particles of that substance.
What is a Gram?
A gram (g) is a unit of mass in the metric system. It represents the base unit for measuring the amount of matter in an object. Unlike moles, which represent a count of particles, grams directly measure the physical weight or mass.
The Bridge: Molar Mass
The key to converting between moles and grams is the molar mass. Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It's essentially the atomic mass (or molecular mass for compounds) expressed in grams. You can find molar mass values on the periodic table for individual elements or calculate it for compounds using the atomic masses of their constituent elements.
Converting Moles to Grams: The Formula
The fundamental formula for converting moles to grams is:
Mass (in grams) = Moles x Molar Mass (g/mol)
This formula elegantly connects the number of particles (moles) to the physical mass (grams) of a substance. Let's break down how to use it effectively.
Step-by-Step Guide
-
Identify the Substance: First, clearly identify the chemical substance you're working with. This is crucial because the molar mass is specific to each substance.
-
Determine the Molar Mass: Find the molar mass of the substance. For elements, this is simply their atomic mass from the periodic table. For compounds, you'll need to add the atomic masses of all the atoms in the chemical formula. For instance, the molar mass of water (H₂O) is calculated as follows:
- Hydrogen (H): 1.008 g/mol x 2 = 2.016 g/mol
- Oxygen (O): 16.00 g/mol
- Total: 2.016 g/mol + 16.00 g/mol = 18.016 g/mol
-
Input Values into the Formula: Once you have the number of moles and the molar mass, plug them into the formula: Mass (g) = Moles x Molar Mass (g/mol)
-
Calculate the Mass: Perform the multiplication to obtain the mass in grams.
Examples: From Simple to Complex
Let's work through several examples to illustrate the conversion process.
Example 1: Converting Moles of a Single Element
Problem: How many grams are there in 2.5 moles of carbon (C)?
Solution:
- Substance: Carbon (C)
- Molar Mass: The atomic mass of carbon from the periodic table is approximately 12.01 g/mol.
- Calculation: Mass (g) = 2.5 mol x 12.01 g/mol = 30.025 g
Answer: There are 30.025 grams in 2.5 moles of carbon.
Example 2: Converting Moles of a Compound
Problem: How many grams are there in 0.75 moles of sodium chloride (NaCl)?
Solution:
- Substance: Sodium chloride (NaCl)
- Molar Mass:
- Sodium (Na): 22.99 g/mol
- Chlorine (Cl): 35.45 g/mol
- Total: 22.99 g/mol + 35.45 g/mol = 58.44 g/mol
- Calculation: Mass (g) = 0.75 mol x 58.44 g/mol = 43.83 g
Answer: There are 43.83 grams in 0.75 moles of sodium chloride.
Example 3: A More Complex Compound
Problem: Calculate the mass in grams of 1.2 moles of sulfuric acid (H₂SO₄).
Solution:
- Substance: Sulfuric acid (H₂SO₄)
- Molar Mass:
- Hydrogen (H): 1.008 g/mol x 2 = 2.016 g/mol
- Sulfur (S): 32.07 g/mol
- Oxygen (O): 16.00 g/mol x 4 = 64.00 g/mol
- Total: 2.016 g/mol + 32.07 g/mol + 64.00 g/mol = 98.086 g/mol
- Calculation: Mass (g) = 1.2 mol x 98.086 g/mol = 117.70 g (approximately)
Answer: There are approximately 117.70 grams in 1.2 moles of sulfuric acid.
Common Mistakes and How to Avoid Them
Several common errors can occur during mole-to-gram conversions. Let's address them:
-
Incorrect Molar Mass Calculation: Double-check your molar mass calculation. A small error here will propagate through the entire calculation. Pay close attention to subscripts in chemical formulas.
-
Unit Errors: Always include units in your calculations. This helps prevent errors and ensures you're using the correct units in the formula.
-
Significant Figures: Be mindful of significant figures. Your final answer should reflect the precision of your input values.
-
Misinterpreting the Question: Carefully read and understand the problem statement. Make sure you are converting moles to grams, and not vice versa.
Beyond the Basics: Applications in Chemistry
The ability to convert moles to grams is fundamental to many areas of chemistry, including:
-
Stoichiometry: Stoichiometry deals with the quantitative relationships between reactants and products in chemical reactions. Converting between moles and grams is essential for calculating yields, limiting reactants, and determining the amount of products formed.
-
Solution Chemistry: When working with solutions (mixtures of solutes in solvents), converting moles to grams is necessary to determine the mass of solute needed to prepare a solution of a specific concentration.
-
Titrations: Titrations involve the gradual addition of a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte). Molar mass conversions are crucial for calculating the concentration of the analyte.
-
Gas Laws: The ideal gas law (PV=nRT) relates pressure, volume, temperature, and the number of moles of a gas. Converting moles to grams allows you to relate the physical properties of a gas to its mass.
Conclusion
Converting moles to grams is a cornerstone skill in chemistry. Mastering this conversion empowers you to tackle a wide range of chemical calculations and deepen your understanding of chemical principles. By following the step-by-step guide, practicing with diverse examples, and being aware of potential pitfalls, you can confidently navigate mole-to-gram conversions and excel in your chemistry studies. Remember, practice is key – the more you work through examples, the more comfortable and accurate you will become.
Latest Posts
Latest Posts
-
Four Letter Words From A To Z
Mar 10, 2025
-
How Big Is 5cm By 5cm
Mar 10, 2025
-
5 Letter Words Ending With On
Mar 10, 2025
-
Which Of These Statements Is True
Mar 10, 2025
-
What Is 2 5 As A Percent
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about How Do You Change Moles To Grams . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
