Horizontal Rows On The Periodic Table Are Called
Juapaving
Mar 12, 2025 · 6 min read
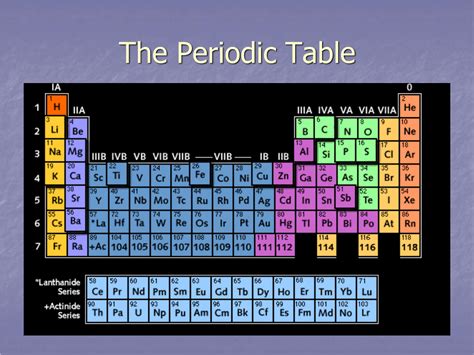
Table of Contents
Horizontal Rows on the Periodic Table are Called: Periods – A Deep Dive into Periodic Trends
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Understanding its organization is crucial for comprehending chemical behavior. A frequently asked question, especially for students beginning their chemistry journey, is: what are the horizontal rows on the periodic table called? The answer is periods. But understanding why they are called periods and what they reveal about elemental properties requires a deeper exploration. This article delves into the intricacies of periods, exploring their significance in predicting and understanding periodic trends.
Understanding Periods: More Than Just a Row
The horizontal rows on the periodic table, known as periods, represent elements with the same number of electron shells. This seemingly simple statement holds immense significance in determining an element's chemical and physical properties. Each period begins with an element having a single electron in a new electron shell, and ends with a noble gas, characterized by a completely filled outermost electron shell. This consistent pattern of electron shell filling is the foundation of the periodic table's structure and the reason for the observed periodic trends.
Electron Shells and Periodicity: The Key Relationship
The number of the period directly corresponds to the principal quantum number (n) of the element's outermost electron shell. For example, elements in period 1 (hydrogen and helium) have electrons only in the n=1 shell, while elements in period 2 (lithium to neon) have electrons in the n=1 and n=2 shells. This progression continues across subsequent periods, with each period adding a new principal energy level. This fundamental relationship between period number and electron shells is what drives the recurring patterns of properties we observe moving across and down the periodic table. Understanding this is crucial to comprehending periodic trends.
Periodic Trends and Their Connection to Periods
The arrangement of elements in periods allows us to predict and understand several crucial periodic trends, including:
1. Atomic Radius: Across and Down the Table
Atomic radius, the distance from the nucleus to the outermost electron, shows a clear trend within periods. As we move from left to right across a period, the atomic radius generally decreases. This is because the number of protons in the nucleus increases, resulting in a stronger attraction to the electrons, pulling them closer to the nucleus. The added electrons are filling the same principal energy level, not adding a new shell that would increase the atomic radius.
Conversely, moving down a group (vertical column), the atomic radius increases. This is because each subsequent period adds a new electron shell, placing the outermost electrons further from the nucleus.
2. Ionization Energy: The Energy to Remove an Electron
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. As we move across a period, the ionization energy generally increases. This is directly related to the increasing nuclear charge and decreasing atomic radius. The stronger the attraction between the nucleus and the electrons, the more energy is required to remove an electron.
Similarly, moving down a group, ionization energy generally decreases. The increased distance between the nucleus and the outermost electrons (due to the larger atomic radius) weakens the attraction, making it easier to remove an electron.
3. Electronegativity: A Tug-of-War for Electrons
Electronegativity measures an atom's ability to attract electrons in a chemical bond. Like ionization energy, electronegativity generally increases across a period, due to the increasing nuclear charge and decreasing atomic radius. Elements on the right side of the table have a greater pull on electrons in a bond.
Moving down a group, electronegativity generally decreases. The increased distance between the nucleus and the outermost electrons reduces the atom's ability to attract electrons in a bond.
4. Metallic Character: From Metals to Nonmetals
Metallic character refers to the properties typically associated with metals, such as conductivity, malleability, and ductility. Across a period, metallic character generally decreases, transitioning from metals on the left to nonmetals on the right. This reflects the increasing tendency for elements to gain electrons and form negative ions rather than lose electrons and form positive ions.
Moving down a group, metallic character generally increases. Elements at the bottom of the groups exhibit stronger metallic characteristics.
Periods and the Prediction of Chemical Behavior
The periodic trends discussed above are not merely academic exercises. They are powerful tools for predicting the chemical behavior of elements. By understanding the position of an element within a period, we can anticipate its reactivity, bonding preferences, and the types of compounds it's likely to form. For instance, knowing that electronegativity increases across a period allows us to predict the polarity of a bond formed between elements from different sides of a period.
The predictability of these trends is a testament to the power of the periodic table's organization. It demonstrates that the arrangement of elements is not arbitrary; it reflects fundamental principles of atomic structure and electron configuration.
Beyond the Basics: A Deeper Look into Periodicity
While the basic trends mentioned above provide a good starting point, the relationship between periods and elemental properties is more nuanced than simple increases or decreases. Several factors can influence these trends:
-
Shielding Effect: Inner electrons shield outer electrons from the full nuclear charge, reducing the effective nuclear charge experienced by valence electrons. This effect becomes more significant in larger atoms, influencing atomic radius and ionization energy.
-
Electron-Electron Repulsion: Repulsion between electrons in the same shell can slightly counteract the attractive force of the nucleus, affecting atomic radius and other properties.
-
Anomalous Behavior: Certain elements deviate from the general periodic trends, largely due to electron configurations and half-filled or completely filled subshells. For example, some anomalies can be found in the transition metal series.
-
Periodicity of Other Properties: Besides those already mentioned, many other properties show periodicity, such as melting and boiling points, density, and various magnetic properties.
Conclusion: The Enduring Significance of Periods
The horizontal rows on the periodic table, the periods, are far more than just a convenient way to arrange elements. They represent a fundamental aspect of atomic structure – the number of electron shells – and directly influence the chemical and physical properties of the elements. Understanding periods and the periodic trends they reveal is fundamental to comprehending chemical behavior, predicting reactivity, and interpreting the vast wealth of information contained within the periodic table. From predicting the reactivity of an alkali metal to understanding the bonding behavior of nonmetals, the concept of periods provides a key to unlocking the secrets of the chemical world. The seemingly simple concept of periods thus forms the bedrock of modern chemistry, providing a framework for understanding the relationships and behaviors of all known elements.
Latest Posts
Latest Posts
-
Is 19 A Prime Number Or A Composite Number
May 09, 2025
-
Is Force Increase On An Inclined Plane
May 09, 2025
-
Interesting Words That Start With V
May 09, 2025
-
Compare And Contrast A Light Microscope And An Electron Microscope
May 09, 2025
-
What Is 6 25 Meters In Feet And Inches
May 09, 2025
Related Post
Thank you for visiting our website which covers about Horizontal Rows On The Periodic Table Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.