Glucose Starch And Cellulose Are All Examples Of
Juapaving
Mar 14, 2025 · 5 min read
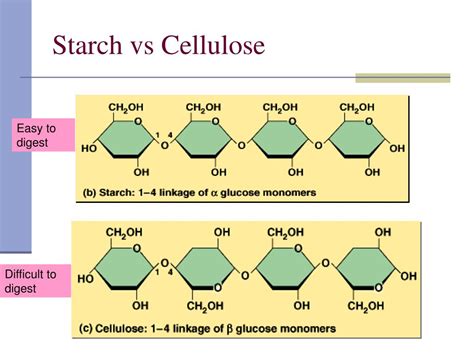
Table of Contents
Glucose, Starch, and Cellulose: All Examples of Carbohydrates
Glucose, starch, and cellulose are all examples of carbohydrates. While they share this fundamental classification, they differ significantly in their structure, function, and digestibility. Understanding these differences is crucial for grasping their roles in biology, nutrition, and various industrial applications. This article delves deep into the chemical makeup, properties, and applications of these vital carbohydrates.
What are Carbohydrates?
Carbohydrates are organic compounds composed of carbon, hydrogen, and oxygen atoms, usually in a ratio of 1:2:1. They are the most abundant biomolecules on Earth and serve as a primary source of energy for living organisms. Carbohydrates are broadly categorized into three types: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides: The Building Blocks
Monosaccharides are the simplest form of carbohydrates, also known as simple sugars. They cannot be further hydrolyzed (broken down) into smaller sugar units. Glucose, fructose, and galactose are common examples. Glucose, in particular, is the most important monosaccharide, serving as the primary energy source for cells. Its structure, a six-carbon ring (hexose), is fundamental to understanding starch and cellulose.
Disaccharides: Two Monosaccharides Unite
Disaccharides are formed when two monosaccharides join together through a glycosidic bond, a type of covalent bond. Sucrose (table sugar), composed of glucose and fructose, is a prime example. Lactose (milk sugar), a combination of glucose and galactose, and maltose (malt sugar), formed from two glucose units, are other important disaccharides.
Polysaccharides: Complex Carbohydrates
Polysaccharides are long chains of monosaccharides linked together by glycosidic bonds. They represent complex carbohydrates and are often insoluble in water. Starch and cellulose are prominent examples of polysaccharides, differing significantly in their structure and function.
Glucose: The Universal Energy Currency
Glucose (C₆H₁₂O₆) is a hexose sugar, meaning it contains six carbon atoms. Its structure exists primarily as a six-membered ring, although it can also exist in an open-chain form. It's highly soluble in water and plays a vital role in cellular respiration, the process by which cells convert glucose into energy in the form of ATP (adenosine triphosphate).
Glucose's Role in Metabolism
Glucose undergoes a series of metabolic reactions, including glycolysis, the Krebs cycle, and oxidative phosphorylation, to generate ATP. This energy powers numerous cellular processes, from muscle contraction to protein synthesis. The body maintains a tight regulation of blood glucose levels to ensure a constant supply of energy.
Glucose Sources and Importance
Glucose is found naturally in fruits, honey, and other sweet foods. It's also a product of the breakdown of starch and other complex carbohydrates during digestion. Maintaining healthy blood glucose levels is critical for preventing diseases such as diabetes.
Starch: The Plant's Energy Storage
Starch is a polysaccharide composed of numerous glucose units linked together. It serves as the primary energy storage molecule in plants. Starch exists in two main forms: amylose and amylopectin.
Amylose: A Linear Chain
Amylose consists of a long, unbranched chain of glucose molecules linked by α-1,4-glycosidic bonds. This linear structure allows for compact packing within plant cells.
Amylopectin: A Branched Chain
Amylopectin, unlike amylose, is a highly branched molecule. It also consists of glucose units linked by α-1,4-glycosidic bonds, but it also contains α-1,6-glycosidic bonds at branch points. This branching pattern allows for rapid enzymatic breakdown and release of glucose when needed.
Starch's Importance in Human Nutrition
Starch is a significant source of dietary energy for humans. During digestion, enzymes break down starch into glucose, which is then absorbed into the bloodstream and utilized by cells. Starchy foods like potatoes, rice, corn, and wheat are staples in many diets worldwide.
Starch's Industrial Applications
Starch also has numerous industrial applications. It is used in the production of paper, textiles, adhesives, and biodegradable plastics. Its ability to thicken liquids makes it a common ingredient in foods, such as sauces and gravies.
Cellulose: The Structural Component of Plants
Cellulose, like starch, is a polysaccharide composed of glucose units. However, the type of glycosidic bond and the resulting structure are dramatically different. Cellulose consists of long, unbranched chains of glucose molecules linked by β-1,4-glycosidic bonds. This seemingly small difference in bonding has profound consequences.
The β-1,4-Glycosidic Bond: Key to Cellulose's Strength
The β-1,4-glycosidic bond in cellulose leads to a linear structure that forms strong hydrogen bonds with adjacent cellulose chains. These hydrogen bonds create highly organized microfibrils, which give cellulose its remarkable strength and structural rigidity. This is what makes cellulose the main structural component of plant cell walls.
Cellulose's Indigestibility
Humans lack the necessary enzymes to break down the β-1,4-glycosidic bonds in cellulose. Therefore, cellulose is largely indigestible by humans, although it plays a vital role as dietary fiber. Fiber promotes regular bowel movements and contributes to overall gut health.
Cellulose's Industrial Applications
Cellulose is used extensively in the production of paper, textiles (cotton, linen), and various other materials. It is also used as a thickener and stabilizer in food products. Cellulose derivatives, such as cellulose acetate, find applications in the manufacture of plastics and films.
Comparing Glucose, Starch, and Cellulose
| Feature | Glucose | Starch | Cellulose |
|---|---|---|---|
| Type | Monosaccharide | Polysaccharide | Polysaccharide |
| Monomer | Glucose | Glucose | Glucose |
| Glycosidic Bond | N/A | α-1,4 (and α-1,6 in amylopectin) | β-1,4 |
| Structure | Single sugar unit | Linear (amylose) & branched (amylopectin) | Linear, highly organized |
| Function | Energy source | Energy storage (plants) | Structural support (plants) |
| Digestibility | Easily digestible | Digestible by humans | Indigestible by humans |
| Solubility | Highly soluble | Partially soluble | Insoluble |
Conclusion
Glucose, starch, and cellulose are all carbohydrates, but their structural differences lead to distinct functions and properties. Glucose serves as the primary energy source for cells. Starch acts as the energy storage molecule in plants and is a key component of the human diet. Cellulose provides structural support in plants and functions as dietary fiber in humans. Understanding the nuances of these three carbohydrates is crucial for comprehending biological processes, nutrition, and various industrial applications. Further research into their properties and applications is ongoing, promising new discoveries and innovations in various fields. From sustainable materials to advanced biofuels, the potential of these ubiquitous carbohydrates is vast and continues to be explored. The ongoing research promises to unlock further potential applications of these essential biomolecules, enhancing our understanding of life and leading to new technologies across numerous sectors.
Latest Posts
Latest Posts
-
Undifferentiated Diploid Spermatogenic Cells Are Called
May 09, 2025
-
Nucleotides Contain A Sugar A Phosphate And A Nitrogenous
May 09, 2025
-
What Are The Two Types Of Mechanical Energy
May 09, 2025
-
Sample Letter Of Refund Payment To Customer
May 09, 2025
-
What Is The Source Of Oxygen Released During Photosynthesis
May 09, 2025
Related Post
Thank you for visiting our website which covers about Glucose Starch And Cellulose Are All Examples Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.