Freezing Point Of Water A. C B. F C. K
Juapaving
Mar 22, 2025 · 7 min read
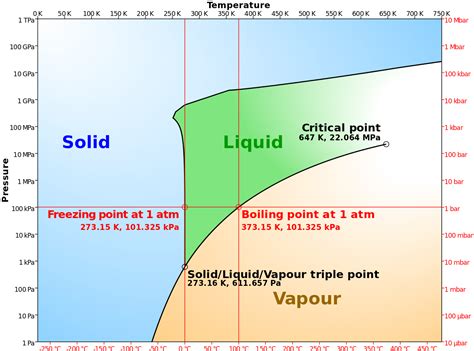
Table of Contents
Freezing Point of Water: A Comprehensive Guide (Celsius, Fahrenheit, Kelvin)
Water, the elixir of life, exhibits a fascinating property: its freezing point. Understanding this seemingly simple concept opens a door to a deeper appreciation of its crucial role in various scientific disciplines, everyday phenomena, and even our survival. This comprehensive guide will delve into the freezing point of water, exploring its values across different temperature scales – Celsius (°C), Fahrenheit (°F), and Kelvin (K) – and examining the underlying scientific principles.
What is the Freezing Point of Water?
The freezing point of water is the temperature at which water transitions from its liquid state to its solid state (ice). This transition, known as freezing or solidification, occurs when the kinetic energy of water molecules decreases to the point where they can no longer overcome the attractive forces holding them together in a crystalline structure. This process is reversible; ice melts back into liquid water when the temperature increases beyond the freezing point.
Crucially, the freezing point of water is not always precisely 0°C (32°F, 273.15 K). Several factors can influence this, including:
- Pressure: While the standard freezing point is at 1 atmosphere of pressure, increasing pressure slightly lowers the freezing point. This is an unusual property of water, unlike most substances.
- Impurities: Dissolved substances, such as salts or sugars, can lower the freezing point of water. This is the principle behind using salt to de-ice roads in winter. The more impurities present, the greater the depression of the freezing point.
- Isotopes: The isotopic composition of water can subtly affect its freezing point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen will freeze at slightly higher temperatures.
Freezing Point in Different Temperature Scales
Understanding the freezing point of water requires familiarity with the three major temperature scales: Celsius, Fahrenheit, and Kelvin. Let's examine the freezing point of water in each:
A. Celsius (°C)
The Celsius scale, also known as the centigrade scale, is a metric system temperature scale. It defines the freezing point of water at 0°C and the boiling point at 100°C at standard atmospheric pressure. This scale is widely used globally for scientific purposes and everyday life.
The Celsius scale is named after Swedish astronomer Anders Celsius, who developed a similar scale in 1742. The scale is linear, meaning that equal intervals on the scale represent equal temperature changes. This makes it convenient for scientific calculations and comparisons.
Why is 0°C chosen as the freezing point? This was a practical choice, establishing a readily observable and easily reproducible reference point for temperature measurement.
B. Fahrenheit (°F)
The Fahrenheit scale is another widely used temperature scale, primarily in the United States. It defines the freezing point of water at 32°F and the boiling point at 212°F at standard atmospheric pressure.
The Fahrenheit scale was developed by German physicist Daniel Gabriel Fahrenheit in 1724. Unlike Celsius, which uses the freezing and boiling points of water as its reference points, Fahrenheit initially used a mixture of ice, water, and ammonium chloride (a freezing mixture) as his zero point, and human body temperature as another reference point. This resulted in a somewhat arbitrary scale.
The conversion between Celsius and Fahrenheit is given by the following formula:
°F = (°C × 9/5) + 32
C. Kelvin (K)
The Kelvin scale is the absolute temperature scale, forming the basis of thermodynamic calculations. It defines absolute zero as 0 K, the theoretical point where all molecular motion ceases. The freezing point of water on the Kelvin scale is 273.15 K.
The Kelvin scale is named after Lord Kelvin (William Thomson), who proposed it in the 19th century. This scale is crucial in many areas of physics and chemistry, especially those involving thermodynamic processes. Because it starts at absolute zero, there are no negative values on the Kelvin scale.
The conversion between Celsius and Kelvin is straightforward:
K = °C + 273.15
The Kelvin scale underscores the concept of absolute temperature, reflecting the actual energy content of a system. This makes it particularly suitable for scientific applications involving energy transfers and phase transitions.
The Significance of Water's Freezing Point
The freezing point of water is far more than just a numerical value; it has profound implications across various aspects of our world:
-
Climate Regulation: The freezing and melting of water plays a vital role in regulating Earth's climate. The high heat capacity of water means that large bodies of water can absorb significant amounts of heat without drastic temperature changes, moderating regional temperatures. The freezing of water in polar regions and high altitudes influences global weather patterns and ocean currents.
-
Ecosystems: The freezing and thawing cycles in various ecosystems significantly impact the survival and behavior of numerous plant and animal species. For example, the formation of ice influences the availability of water resources for plants and animals, and ice cover affects aquatic life.
-
Industry and Technology: The freezing point of water is crucial in many industrial processes. It dictates the conditions for storing and transporting temperature-sensitive materials, designing cooling systems, and controlling chemical reactions. The freezing of water is utilized in food preservation, manufacturing processes, and material science.
-
Human Health: The body's temperature regulation is closely tied to water's properties. Sweating, a process involving water evaporation, helps cool the body. The freezing point of water dictates the temperature at which hypothermia, a potentially life-threatening condition, sets in.
-
Weather Phenomena: The freezing point of water is directly related to many weather phenomena, including snow, ice, frost, and freezing rain. Understanding how temperature influences the state of water is essential for accurate weather forecasting.
Beyond the Simple Freezing Point: Supercooling and Nucleation
While 0°C (32°F, 273.15 K) represents the equilibrium freezing point of water under standard conditions, it's possible for water to remain liquid below this temperature – a phenomenon known as supercooling.
Supercooling happens when the water lacks sufficient nucleation sites – imperfections or irregularities within the liquid – for the crystallization process to begin efficiently. These nucleation sites serve as templates for the formation of ice crystals. In the absence of these sites, the water can remain in a metastable, supercooled state until disturbed or a nucleation site is introduced, triggering rapid freezing.
This supercooling effect has implications for various processes, including cloud formation (where supercooled water droplets can exist in the atmosphere before freezing) and the preservation of biological samples.
The Anomalous Behavior of Water: Why is it Unique?
Water exhibits many anomalous properties, and its freezing point is no exception. Unlike most substances, which become denser upon freezing, ice is less dense than liquid water. This is due to the unique hydrogen bonding in water molecules. In ice, these bonds create a relatively open crystalline structure, leading to a lower density compared to the more closely packed molecules in liquid water. This property is vital for aquatic life, as it allows ice to float on water, preventing bodies of water from freezing solid from the bottom up.
This lower density of ice also impacts various environmental processes, such as the insulation provided by ice cover on lakes and rivers, allowing aquatic life to survive beneath the ice during winter.
Conclusion: The Importance of Understanding the Freezing Point
The freezing point of water is a seemingly simple concept with vast implications across numerous scientific disciplines, environmental processes, and everyday life. Understanding its value across different temperature scales, the factors influencing its precise value, and the unique properties of water surrounding freezing are crucial for comprehending the complexities of our world. From climate regulation to industrial applications and human survival, the freezing point of water holds a fundamental role in shaping our environment and influencing technological advancements. Further research into water's properties, particularly those related to freezing and melting, continues to reveal important insights with far-reaching consequences.
Latest Posts
Latest Posts
-
Which Subatomic Particle Is The Heaviest
Mar 22, 2025
-
What Can 41 Be Divided By
Mar 22, 2025
-
What Is The Difference Between Monarchy And Democracy
Mar 22, 2025
-
Difference Between Pl Sql And Sql
Mar 22, 2025
-
Tell Whether The Angles Are Adjacent Or Vertical
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Freezing Point Of Water A. C B. F C. K . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
