Fourth Period Of The Periodic Table
Juapaving
Mar 21, 2025 · 7 min read
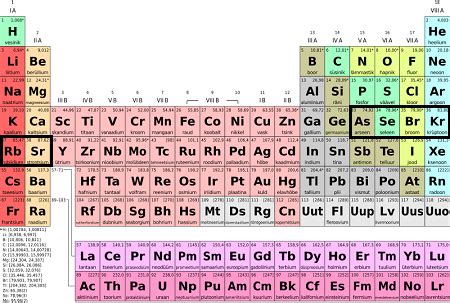
Table of Contents
The Fourth Period: A Deep Dive into the Transition Metals
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. While each period presents unique characteristics, the fourth period holds a particularly significant position, showcasing the introduction of the transition metals and their diverse properties. This period, spanning from Potassium (K) to Krypton (Kr), provides a fascinating case study in the complexities of atomic behavior and its impact on the physical and chemical world. This in-depth exploration will unpack the key features, trends, and exceptions within this crucial period of the periodic table.
Understanding the Fourth Period's Structure
The fourth period distinguishes itself from previous periods by introducing the d-block elements – the transition metals. Unlike the s- and p-block elements, which predominantly exhibit predictable changes in properties along the period, the transition metals display a more nuanced and complex array of behaviors. This is largely due to the variable oxidation states and the involvement of d-electrons in bonding.
The period begins with Potassium (K) and Calcium (Ca), representative of the s-block, continuing the trends of increasing electronegativity and decreasing atomic radius observed in previous periods. However, the introduction of Scandium (Sc) marks the commencement of the d-block, ushering in a new realm of chemical properties. This period concludes with the p-block elements, exhibiting a gradual increase in electronegativity from Gallium (Ga) to Bromine (Br) and culminating in the noble gas Krypton (Kr).
Key Characteristics of the Fourth Period Elements
-
Potassium (K) and Calcium (Ca): These alkaline and alkaline earth metals, respectively, exhibit typical characteristics of their groups – high reactivity, low ionization energies, and the formation of +1 and +2 ions, respectively. Their large atomic radii and low electronegativities contribute to their readiness to lose electrons and form ionic compounds.
-
Scandium (Sc) to Zinc (Zn): This section comprises the first-row transition metals. These elements are characterized by their variable oxidation states, stemming from the ability of d-electrons to participate in bonding. This leads to a wide array of colorful compounds and complex coordination complexes. Their relatively high melting and boiling points compared to s-block elements are attributed to the strong metallic bonding arising from the involvement of d-electrons.
-
Gallium (Ga) to Krypton (Kr): This segment covers the p-block elements, mirroring trends observed in previous periods. The electronegativity gradually increases across the period, resulting in a shift from metallic properties in Gallium to non-metallic properties in Bromine, with Krypton representing the inert noble gas.
Transition Metals: A Detailed Look
The transition metals of the fourth period are the focal point of interest, exhibiting unique chemical and physical properties that set them apart. Let's explore some key aspects:
Variable Oxidation States
The hallmark of transition metals is their capacity to display multiple oxidation states. This arises from the relatively similar energies of the (n-1)d and ns electrons, allowing them to participate in bonding to varying degrees. For instance, iron (Fe) can exist in +2 and +3 oxidation states, leading to compounds like ferrous oxide (FeO) and ferric oxide (Fe₂O₃), exhibiting distinctly different properties. This variability in oxidation states is responsible for the rich and diverse chemistry of transition metal compounds.
Colorful Compounds
Many transition metal compounds exhibit vibrant colors. This is a consequence of the d-d electronic transitions. When light interacts with these compounds, electrons in the d-orbitals can absorb specific wavelengths of light, resulting in the transmission of complementary colors. This phenomenon is responsible for the intense colors observed in many gemstones and pigments. For example, the deep blue color of copper(II) sulfate pentahydrate is a direct result of these d-d transitions.
Catalytic Activity
Transition metals and their compounds are widely used as catalysts in various chemical processes. Their variable oxidation states allow them to readily gain and lose electrons, facilitating the breaking and formation of bonds in reactant molecules. This catalytic ability is crucial in numerous industrial processes, including the Haber-Bosch process for ammonia synthesis and the catalytic converters in automobiles. Platinum, palladium, and nickel are prime examples of transition metals with exceptional catalytic properties.
Magnetic Properties
Several transition metals exhibit magnetic properties due to the presence of unpaired electrons in their d-orbitals. Iron, cobalt, and nickel are well-known examples of ferromagnetic materials, possessing strong magnetic moments due to the parallel alignment of electron spins in their domains. This property is crucial in various applications, including electromagnets, permanent magnets, and magnetic data storage.
Complex Ion Formation
Transition metals have a strong tendency to form complex ions. These complexes consist of a central metal ion surrounded by ligands – molecules or ions that donate electron pairs to the metal ion. The formation of these complexes often leads to significant changes in the properties of the metal ion, including color, stability, and reactivity. The study of coordination chemistry, encompassing the formation and properties of these complex ions, is a vast and important area of inorganic chemistry.
Trends and Exceptions within the Fourth Period
While the fourth period largely follows general periodic trends, some exceptions and irregularities are noteworthy:
Atomic Radius
The atomic radius generally decreases across the period from potassium to krypton, reflecting the increasing nuclear charge and the imperfect shielding of the added electrons by the inner electrons. However, the transition metals show a smaller decrease in atomic radius compared to the s- and p-block elements due to the shielding effect of the d-electrons.
Ionization Energy
Ionization energy, the energy required to remove an electron from an atom, generally increases across the period, reflecting the stronger attraction between the nucleus and electrons. However, some irregularities exist, particularly within the transition metals, due to the subtle variations in electron configurations and shielding effects.
Electronegativity
Electronegativity, the ability of an atom to attract electrons in a chemical bond, generally increases across the period, with the most electronegative element being bromine. The transition metals exhibit a relatively smaller increase in electronegativity compared to the s- and p-block elements.
Melting and Boiling Points
The melting and boiling points of elements generally show a pattern across a period, often with a peak at the transition metals. This is due to the strong metallic bonding contributed by the involvement of d-electrons. The strong interatomic forces lead to higher melting and boiling points compared to the s- and p-block elements. However, there are variations even within the transition metals, dependent on specific electronic configurations and crystal structures.
Applications of Fourth Period Elements
The elements of the fourth period are indispensable in various technological and industrial applications. Let's briefly explore some examples:
-
Potassium (K): Used in fertilizers, as an electrolyte in batteries, and in the production of soaps and detergents.
-
Calcium (Ca): Essential for bone health, employed in construction materials (cement), and as a reducing agent in metallurgy.
-
Iron (Fe): The backbone of the steel industry, crucial for construction, transportation, and countless other applications.
-
Chromium (Cr): Used in stainless steel for its corrosion resistance, in pigments, and in electroplating.
-
Manganese (Mn): Used in steel alloys to enhance strength and hardness, and in batteries.
-
Nickel (Ni): Used in stainless steel, in nickel-cadmium batteries, and as a catalyst.
-
Copper (Cu): An excellent conductor of electricity, used extensively in electrical wiring and electronics.
-
Zinc (Zn): Used in galvanization to protect iron from corrosion, in brass, and in batteries.
-
Gallium (Ga): Used in semiconductors, in LEDs, and in high-temperature thermometers.
-
Bromine (Br): Used in flame retardants, in pesticides, and in photography.
Conclusion
The fourth period of the periodic table represents a pivotal stage, introducing the complexities of transition metals and their profound influence on chemical and physical properties. Understanding the trends, exceptions, and applications of these elements is fundamental to comprehending a vast range of scientific and technological advancements. From the vibrant colors of coordination complexes to the catalytic prowess of transition metals, this period encapsulates the beauty and intricacy of the periodic system's organization. Further exploration into the specific characteristics of each element within the fourth period can provide deeper insights into the diverse chemical landscape they shape. The fourth period's elements are not merely entries on a chart; they are the building blocks of countless essential materials, impacting our daily lives in myriad ways.
Latest Posts
Latest Posts
-
How Do You Spell The Word 18
Mar 22, 2025
-
What Do Arrows Represent In A Food Chain
Mar 22, 2025
-
Can Gas Turn Into A Liquid
Mar 22, 2025
-
What Organelle In A Plant Is Chlorophyll Found In
Mar 22, 2025
-
Takes The Shape Of Its Container
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Fourth Period Of The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
