Enthalpy Of Ch4 2o2--- Co2 2h2o
Juapaving
May 11, 2025 · 6 min read
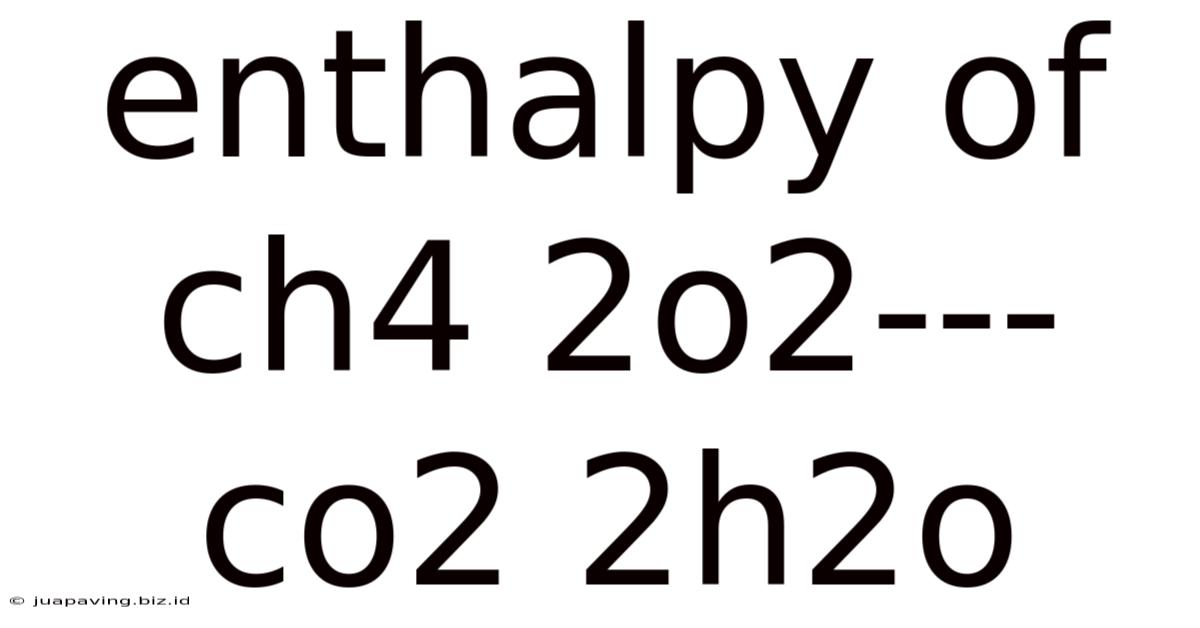
Table of Contents
Enthalpy of Combustion: Delving Deep into CH₄ + 2O₂ → CO₂ + 2H₂O
The combustion of methane (CH₄), a primary component of natural gas, is a fundamental chemical reaction with significant implications for energy production and climate change. Understanding the enthalpy change (ΔH) associated with this reaction, represented by the equation CH₄ + 2O₂ → CO₂ + 2H₂O, is crucial for various applications, from calculating energy output in power plants to modeling atmospheric processes. This article will delve into the intricacies of this reaction, exploring the concept of enthalpy, its calculation methods, factors influencing its value, and its broader significance.
Understanding Enthalpy and Enthalpy of Combustion
Enthalpy (H) is a thermodynamic property representing the total heat content of a system at constant pressure. It's a state function, meaning its value depends only on the initial and final states of the system, not the path taken. In chemical reactions, the change in enthalpy (ΔH) indicates whether the reaction is exothermic (releases heat, ΔH < 0) or endothermic (absorbs heat, ΔH > 0).
Enthalpy of combustion (ΔHc) specifically refers to the enthalpy change when one mole of a substance undergoes complete combustion in excess oxygen under standard conditions (typically 298 K and 1 atm). For methane, the reaction is:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(l)
This reaction is highly exothermic, meaning a significant amount of heat is released. This released heat is harnessed in various applications, making methane a valuable fuel source.
Calculating the Enthalpy of Combustion
Several methods exist for determining the enthalpy of combustion of methane:
1. Experimental Measurement using Calorimetry: This is the most direct method. A known mass of methane is burned in a calorimeter, a device designed to measure heat transfer. The temperature change of the calorimeter and its contents is used to calculate the heat released, which is then converted to enthalpy change per mole of methane.
2. Using Standard Enthalpies of Formation: Hess's Law allows us to calculate the enthalpy of combustion indirectly using the standard enthalpies of formation (ΔHf°) of the reactants and products. Standard enthalpy of formation is the enthalpy change when one mole of a compound is formed from its constituent elements in their standard states. Hess's Law states that the total enthalpy change for a reaction is independent of the pathway taken. Therefore:
ΔHc° = ΣΔHf°(products) - ΣΔHf°(reactants)
For the combustion of methane:
ΔHc° = [ΔHf°(CO₂) + 2ΔHf°(H₂O)] - [ΔHf°(CH₄) + 2ΔHf°(O₂)]
Since the standard enthalpy of formation of an element in its standard state is zero (ΔHf°(O₂) = 0), the equation simplifies to:
ΔHc° = ΔHf°(CO₂) + 2ΔHf°(H₂O) - ΔHf°(CH₄)
By substituting the known standard enthalpies of formation for CO₂, H₂O, and CH₄ (obtained from thermodynamic tables), we can calculate the standard enthalpy of combustion for methane.
3. Bond Energies: This method estimates the enthalpy change by considering the energy required to break the bonds in the reactants and the energy released when new bonds are formed in the products. The enthalpy change is approximated as the difference between the total bond energies broken and the total bond energies formed. While less precise than calorimetry or using standard enthalpies of formation, this method provides a useful estimate.
Factors Influencing the Enthalpy of Combustion
Several factors can subtly influence the measured enthalpy of combustion:
-
State of Water: The enthalpy of combustion value changes depending on whether the water produced is in liquid or gaseous form. The equation above assumes liquid water (H₂O(l)). If water is in gaseous form (H₂O(g)), the enthalpy of combustion will be less negative because energy is required to vaporize the water.
-
Temperature and Pressure: While standard conditions (298 K and 1 atm) are typically used, variations in temperature and pressure will affect the enthalpy of combustion. Higher temperatures generally lead to slightly higher enthalpy values.
-
Purity of Reactants: Impurities in the methane sample can affect the measured enthalpy of combustion. Precise measurements require highly pure reactants.
-
Incomplete Combustion: If combustion is incomplete (due to insufficient oxygen supply), the products will include carbon monoxide (CO) and/or soot (C), significantly altering the enthalpy change. Complete combustion ensures the formation of only CO₂ and H₂O.
Significance of the Enthalpy of Combustion of Methane
The enthalpy of combustion of methane holds immense significance in several fields:
-
Energy Production: The large negative enthalpy change signifies that a considerable amount of heat is released during the combustion of methane. This heat is harnessed in power plants to generate electricity, and in industrial processes requiring heat.
-
Climate Change: The combustion of methane contributes significantly to greenhouse gas emissions. Carbon dioxide, a potent greenhouse gas, is the primary product of the reaction. Understanding the enthalpy of combustion is essential for evaluating the environmental impact of methane use and developing strategies for mitigating climate change.
-
Thermodynamic Calculations: The enthalpy of combustion is a crucial parameter in various thermodynamic calculations, including equilibrium constant calculations and spontaneity predictions.
-
Fuel Efficiency: Knowing the enthalpy of combustion allows for accurate assessment of the fuel efficiency of methane-based energy sources. This information is critical for designing efficient combustion engines and optimizing energy utilization.
-
Chemical Engineering: In chemical engineering, the enthalpy of combustion is essential in designing and optimizing chemical processes involving methane.
Beyond the Basics: Exploring Related Concepts
The enthalpy of combustion is closely related to other important thermodynamic concepts:
-
Heat of Reaction: This is a broader term referring to the heat transferred during any chemical reaction, while enthalpy of combustion specifically refers to the heat released during combustion.
-
Standard Enthalpy Change: The enthalpy change under standard conditions (298 K and 1 atm).
-
Internal Energy Change: This represents the change in the internal energy of a system, which is related to enthalpy by the equation: ΔH = ΔU + PΔV, where P is pressure and V is volume.
-
Gibbs Free Energy: This determines the spontaneity of a reaction and is related to enthalpy and entropy (S) by the equation: ΔG = ΔH - TΔS, where T is temperature.
Conclusion
The enthalpy of combustion for the reaction CH₄ + 2O₂ → CO₂ + 2H₂O is a fundamental thermodynamic property with far-reaching implications. Accurate determination of this value, through experimental measurements or calculations using standard enthalpies of formation, is crucial for energy production, climate change modeling, and numerous chemical engineering applications. A deeper understanding of this reaction, encompassing the influence of various factors and related thermodynamic concepts, is essential for developing sustainable energy strategies and mitigating the environmental impact of fossil fuel combustion. Further research and technological advancements continue to refine our understanding of this vital reaction and its implications for the future.
Latest Posts
Latest Posts
-
17 Rounded To The Nearest Tenth
May 12, 2025
-
Label The Structures Of The Spinal Cord
May 12, 2025
-
Is The O H Bond Polar Or Nonpolar
May 12, 2025
-
What Percentage Of The Human Body Is Made Of Water
May 12, 2025
-
What Is 4 25 As A Fraction
May 12, 2025
Related Post
Thank you for visiting our website which covers about Enthalpy Of Ch4 2o2--- Co2 2h2o . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.