Elements In Group 17 Are Called
Juapaving
Mar 05, 2025 · 6 min read
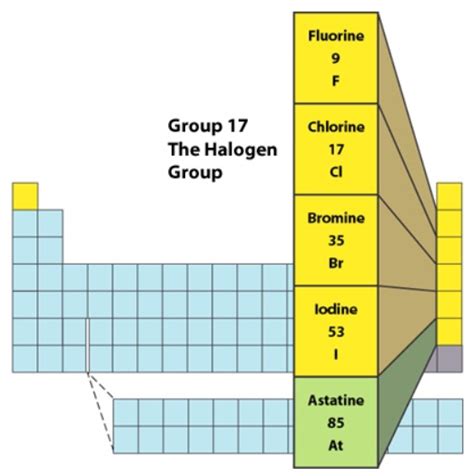
Table of Contents
Elements in Group 17 are Called Halogens: A Deep Dive into their Properties, Reactions, and Applications
Elements in Group 17 of the periodic table are known as halogens. This fascinating group of nonmetals displays a remarkable range of properties and reactivities, making them crucial in various industrial and biological processes. Understanding their characteristics is key to appreciating their significance in chemistry and beyond. This article delves into the fascinating world of halogens, exploring their properties, reactions, and widespread applications.
What are Halogens?
The term "halogen" originates from Greek, where "halos" means "salt" and "genes" means "forming." This aptly describes their primary characteristic: their tendency to react with metals to form salts. The group comprises five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). These elements share several common traits, although variations exist due to their increasing atomic size and electronegativity down the group.
Key Characteristics of Halogens:
- Nonmetals: Halogens are all nonmetals, exhibiting characteristic nonmetallic properties such as poor electrical and thermal conductivity.
- Highly Reactive: Their high electronegativity makes them exceptionally reactive, readily accepting electrons to achieve a stable octet configuration. This reactivity decreases down the group, with fluorine being the most reactive and astatine the least.
- Diatomic Molecules: Halogens exist as diatomic molecules (e.g., F₂, Cl₂, Br₂, I₂) in their elemental state, meaning they exist as pairs of atoms bonded together. This is due to their strong tendency to achieve a stable electron configuration.
- Variable Oxidation States: Halogens can exhibit variable oxidation states in their compounds, primarily -1, +1, +3, +5, and +7. Fluorine, however, almost exclusively shows a -1 oxidation state due to its exceptionally high electronegativity.
- Color and Physical State: The halogens show a distinct trend in their physical states and colors. Fluorine is a pale yellow gas, chlorine is a greenish-yellow gas, bromine is a reddish-brown liquid, and iodine is a dark grey-purple solid. Astatine is a radioactive solid.
Detailed Examination of Individual Halogens:
Let's delve deeper into the unique properties and applications of each halogen:
1. Fluorine (F): The Most Reactive Halogen
Fluorine, the lightest halogen, is a pale yellow, highly reactive gas. Its exceptionally high electronegativity makes it the most reactive element in the periodic table. This reactivity has implications for its handling and applications:
- Applications: Fluorine is used extensively in the production of fluorocarbons, which have diverse applications including refrigerants (although their use is declining due to environmental concerns), polymers like Teflon (polytetrafluoroethylene), and in the semiconductor industry. It is also used in the production of uranium hexafluoride (UF₆) for nuclear fuel enrichment.
- Reactivity: Its high reactivity necessitates specialized handling procedures due to its potential to react violently with many materials.
- Biological Significance: Fluoride ions (F⁻) play a crucial role in preventing dental caries (tooth decay) by strengthening tooth enamel. However, excessive fluoride intake can lead to fluorosis.
2. Chlorine (Cl): A Versatile Element
Chlorine, a greenish-yellow gas, is widely used in various industrial and household applications.
- Applications: Chlorine is primarily used in the production of water purification, disinfecting drinking water and swimming pools. It’s a key component in the production of polyvinyl chloride (PVC), a widely used plastic. Chlorine is also used in the manufacturing of various chemicals, including solvents and pesticides.
- Reactivity: While less reactive than fluorine, chlorine is still a potent oxidizing agent and reacts readily with many substances.
- Biological Significance: While essential in small amounts for some biological processes, chlorine in high concentrations is toxic.
3. Bromine (Br): The Only Liquid Halogen
Bromine is a reddish-brown liquid at room temperature, a unique characteristic among the halogens.
- Applications: Bromine is used in the production of flame retardants, agricultural chemicals, and pharmaceuticals. It is also used in photographic film and dyes.
- Reactivity: Bromine is less reactive than fluorine and chlorine, but still a powerful oxidizing agent.
- Environmental Concerns: Some bromine-containing compounds have raised environmental concerns due to their potential to contribute to ozone depletion and persist in the environment.
4. Iodine (I): Essential for Human Health
Iodine is a dark grey-purple solid that sublimes (transitions directly from solid to gas) at room temperature.
- Applications: Iodine is crucial in human health, as it is a component of thyroid hormones. Iodine deficiency can lead to goiter. It is also used as a disinfectant and in various chemical processes.
- Reactivity: Iodine is the least reactive of the common halogens.
- Biological Significance: Iodine's role in thyroid hormone production is essential for proper metabolism and growth. Iodine deficiency can lead to serious health problems.
5. Astatine (At): The Radioactive Halogen
Astatine is a radioactive element with no stable isotopes. Its short half-life makes its study challenging and limits its practical applications.
Chemical Reactions of Halogens:
Halogens undergo a range of characteristic reactions, primarily driven by their high electronegativity and tendency to gain electrons.
1. Reactions with Metals:
Halogens readily react with most metals to form metal halides. For instance, the reaction of sodium (Na) with chlorine (Cl₂) produces sodium chloride (NaCl), common table salt:
2Na(s) + Cl₂(g) → 2NaCl(s)
The reactivity with metals generally decreases down the group.
2. Reactions with Nonmetals:
Halogens can also react with some nonmetals, forming covalent compounds. For example, chlorine reacts with hydrogen to form hydrogen chloride (HCl), a strong acid:
H₂(g) + Cl₂(g) → 2HCl(g)
3. Displacement Reactions:
Halogens can displace less reactive halogens from their compounds. For example, chlorine can displace bromine from potassium bromide:
Cl₂(g) + 2KBr(aq) → 2KCl(aq) + Br₂(l)
Applications of Halogens:
The applications of halogens span numerous industries and fields:
- Water Purification: Chlorine is widely used as a disinfectant to purify water, making it safe for drinking and other uses.
- Plastics Production: Chlorine is essential in the production of PVC, a versatile plastic used in various applications.
- Refrigerants: Although their use is decreasing, fluorocarbons were once widely used as refrigerants. New refrigerants with less environmental impact are now being developed.
- Flame Retardants: Bromine-containing compounds are used as flame retardants in various materials.
- Pharmaceuticals: Halogens and their compounds are used in the synthesis of numerous pharmaceuticals.
- Dyes and Pigments: Iodine and bromine compounds are used in the production of dyes and pigments.
- Photography: Silver halides are crucial components in photographic film.
- Nuclear Industry: Fluorine is used in the enrichment of uranium for nuclear fuel.
Environmental Concerns and Safety Precautions:
While halogens have numerous beneficial applications, some raise environmental concerns:
- Ozone Depletion: Some fluorocarbons were found to contribute to ozone depletion in the stratosphere. The Montreal Protocol phased out the production of these ozone-depleting substances.
- Persistent Organic Pollutants (POPs): Certain halogenated organic compounds are persistent organic pollutants, meaning they persist in the environment for a long time and can bioaccumulate in organisms.
- Toxicity: Many halogen compounds are toxic, requiring careful handling and disposal.
Safety precautions when working with halogens include proper ventilation, protective clothing, and adherence to relevant safety regulations.
Conclusion:
The halogens, elements in Group 17, are a fascinating group of nonmetals with a wide range of properties and applications. Their high reactivity makes them indispensable in various industrial processes, while their presence in biological systems highlights their significance in life processes. Understanding their properties and potential environmental impacts is crucial for responsible use and development of sustainable alternatives where necessary. Further research continues to unveil new applications and address environmental concerns related to these versatile elements. The continued study of halogens will undoubtedly contribute to advancements in various scientific and technological fields.
Latest Posts
Latest Posts
-
What Is 3 10 As A Percent
Mar 06, 2025
-
How Many Chambers Does The Heart Of A Frog Have
Mar 06, 2025
-
What Is The Gcf Of 42 And 28
Mar 06, 2025
-
The Division Of The Nucleus Is Called
Mar 06, 2025
-
Highest Common Factor Of 8 And 16
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Elements In Group 17 Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
