Elements Are Arranged In The Periodic Table According To Their
Juapaving
Mar 14, 2025 · 6 min read
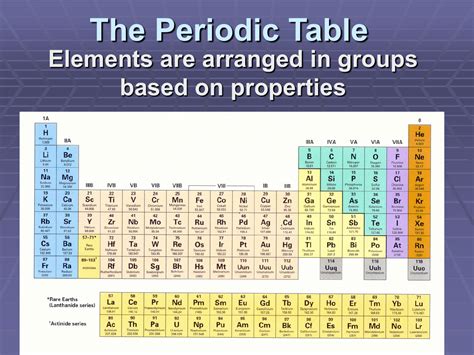
Table of Contents
Elements Are Arranged in the Periodic Table According to Their Atomic Number and Electron Configuration: A Deep Dive
The periodic table, that iconic chart adorning countless science classrooms, isn't just a random collection of elements. Its organization is a testament to the profound understanding of atomic structure and chemical behavior. Elements are arranged not haphazardly, but according to their atomic number and the resulting electron configuration. This arrangement reveals fascinating patterns and predicts the properties of elements with remarkable accuracy. This article will delve into the intricacies of this organization, exploring the underlying principles and the consequences of this ingenious system.
The Foundation: Atomic Number and Electron Configuration
The cornerstone of the periodic table's organization is the atomic number. This number, represented by the symbol Z, signifies the number of protons in an atom's nucleus. Since atoms are electrically neutral, the atomic number also equals the number of electrons orbiting the nucleus. These electrons are not randomly distributed; they occupy specific energy levels or shells surrounding the nucleus.
Energy Levels and Sublevels
Electrons within an atom exist in distinct energy levels, often visualized as concentric shells around the nucleus. The closer the shell is to the nucleus, the lower its energy. Each energy level can accommodate a specific number of electrons: the first shell holds a maximum of two electrons, the second shell eight, and so on. This maximum capacity is given by the formula 2n², where 'n' is the principal quantum number (shell number).
However, the story doesn't end there. Within each energy level (except the first), electrons are further divided into sublevels or subshells. These subshells are designated by the letters s, p, d, and f, each with its own unique shape and capacity. The s subshell can hold a maximum of two electrons, the p subshell six, the d subshell ten, and the f subshell fourteen.
Electron Configuration: The Key to Understanding Periodicity
The electron configuration of an atom describes how its electrons are distributed among these energy levels and subshells. This configuration is crucial in determining an element's chemical properties. For example, the electron configuration of sodium (Na) is 1s²2s²2p⁶3s¹. This tells us that sodium has two electrons in the first shell (1s²), eight electrons in the second shell (2s²2p⁶), and one electron in the third shell (3s¹). It is this outermost electron in the 3s subshell that dictates sodium's reactivity.
The Periodic Table's Structure: Periods and Groups
The periodic table is organized into rows called periods and columns called groups or families. The arrangement reflects the systematic filling of electron shells and subshells as the atomic number increases.
Periods: Reflecting Electron Shells
Each period corresponds to a principal energy level. Elements in the same period have their outermost electrons in the same principal energy level. For instance, all elements in the second period (Li, Be, B, C, N, O, F, Ne) have their outermost electrons in the second energy level (n=2). As we move across a period, the number of electrons in the outermost shell increases until it's filled, leading to a noble gas configuration.
Groups: Reflecting Valence Electrons and Chemical Properties
Elements in the same group share similar chemical properties because they have the same number of valence electrons. Valence electrons are the electrons in the outermost energy level. These electrons participate in chemical bonding, dictating an element's reactivity and the types of compounds it forms.
- Group 1 (Alkali Metals): These elements have one valence electron, making them highly reactive and readily forming +1 ions.
- Group 2 (Alkaline Earth Metals): With two valence electrons, these metals are also reactive, forming +2 ions.
- Group 17 (Halogens): These nonmetals have seven valence electrons and are highly reactive, readily gaining one electron to form -1 ions.
- Group 18 (Noble Gases): These elements have a full outer electron shell (eight electrons, except helium with two), making them exceptionally stable and unreactive.
The similarities in chemical behavior within a group are a direct consequence of their identical valence electron configurations.
Beyond the Basics: Transition Metals, Lanthanides, and Actinides
The periodic table's organization extends beyond the simple filling of s and p subshells. The d and f subshells introduce further complexities.
Transition Metals: The d-block
The transition metals occupy the central block of the periodic table (d-block). These elements are characterized by the filling of the d subshell. They exhibit variable oxidation states, meaning they can lose different numbers of electrons to form ions with different charges. This versatility leads to a wide range of chemical behaviors and colorful compounds.
Lanthanides and Actinides: The f-block
At the bottom of the periodic table are the lanthanides and actinides, also known as the inner transition metals. These elements represent the filling of the f subshell. They are chemically similar within their respective series due to their similar electron configurations. Many actinides are radioactive.
Predicting Properties: The Power of the Periodic Table
The periodic table's organization isn't just a convenient arrangement; it's a powerful tool for predicting the properties of elements.
Trends in Properties
Several important properties exhibit predictable trends across the periodic table:
- Atomic Radius: Generally increases down a group (due to the addition of electron shells) and decreases across a period (due to increased nuclear charge pulling electrons closer).
- Ionization Energy: The energy required to remove an electron. Generally decreases down a group and increases across a period.
- Electronegativity: The ability of an atom to attract electrons in a chemical bond. Generally decreases down a group and increases across a period.
- Metallic Character: The tendency of an element to lose electrons and form positive ions. Generally increases down a group and decreases across a period.
These trends are directly linked to the electron configurations and the effective nuclear charge experienced by the outermost electrons.
The Periodic Law: The Underlying Principle
The entire system is governed by the periodic law, which states that the properties of elements are a periodic function of their atomic numbers. This means that as we increase the atomic number, the properties of elements repeat in a predictable manner. This periodicity is a direct consequence of the periodic filling of electron shells and subshells.
Conclusion: A Testament to Scientific Understanding
The periodic table's organization, based on atomic number and electron configuration, is a remarkable achievement of scientific understanding. Its structure not only provides a systematic classification of elements but also reveals fundamental relationships between their properties and their atomic structure. This elegant arrangement serves as a powerful predictive tool, enabling scientists to understand and anticipate the chemical behavior of elements, paving the way for countless scientific discoveries and technological advancements. The periodic table remains an essential tool in chemistry and related fields, a testament to the power of scientific inquiry and the beauty of underlying natural order. Its continued use and refinement reflect its enduring importance in shaping our comprehension of the material world. Further research continues to unravel the finer details of atomic interactions, leading to a deeper appreciation for the principles that govern the periodic table and the remarkable properties it reveals. The systematic organization of elements allows us to not only classify but also predict their behaviour and potential applications, furthering our understanding of chemical reactions and fostering advancements in diverse fields. The periodic table’s enduring relevance emphasizes its pivotal role in the scientific community.
Latest Posts
Latest Posts
-
A Food Chain Starts With A
May 09, 2025
-
Which Of The Following Statements About Chlorophyll Is Correct
May 09, 2025
-
Kinetic Energy Is Energy An Object Has Because Of Its
May 09, 2025
-
What Is The Least Common Multiple Of 20 And 40
May 09, 2025
-
48 Inches Is What In Feet
May 09, 2025
Related Post
Thank you for visiting our website which covers about Elements Are Arranged In The Periodic Table According To Their . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.