Electron Configuration For First 20 Elements
Juapaving
Apr 06, 2025 · 6 min read
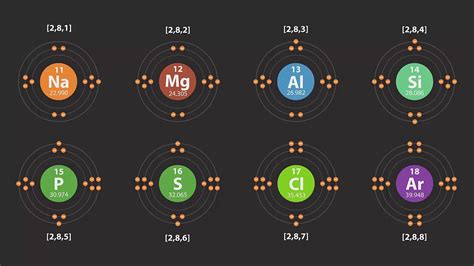
Table of Contents
Electron Configuration for the First 20 Elements: A Comprehensive Guide
Understanding electron configuration is fundamental to grasping the behavior of elements and their place within the periodic table. This comprehensive guide delves into the electron configurations of the first 20 elements, explaining the principles behind their arrangement and how this configuration dictates their chemical properties. We'll explore the rules governing electron placement, delve into the significance of orbitals and subshells, and ultimately, provide a solid foundation for understanding more complex atomic structures.
Understanding the Basics: Orbitals, Subshells, and Shells
Before we dive into specific element configurations, let's establish some key terminology:
-
Shells: Electrons reside in energy levels called shells. These shells are represented by the principal quantum number (n), where n = 1 represents the shell closest to the nucleus (lowest energy), n = 2 is the next shell, and so on. The higher the value of n, the higher the energy level and the greater the distance from the nucleus.
-
Subshells: Within each shell, there are subshells, which are regions of space where electrons are most likely to be found. These subshells are designated by letters: s, p, d, and f. Each subshell has a specific number of orbitals.
-
Orbitals: Orbitals are specific regions within a subshell where a maximum of two electrons can reside, according to the Pauli Exclusion Principle (which states that no two electrons in an atom can have the same set of four quantum numbers).
Here's a summary of the subshells, their orbital capacity, and the maximum number of electrons they can hold:
- s subshell: One orbital, holding a maximum of 2 electrons.
- p subshell: Three orbitals, holding a maximum of 6 electrons.
- d subshell: Five orbitals, holding a maximum of 10 electrons.
- f subshell: Seven orbitals, holding a maximum of 14 electrons.
The Aufbau Principle and Hund's Rule: Filling the Orbitals
The arrangement of electrons in an atom follows specific rules:
-
Aufbau Principle: Electrons fill the lowest energy levels first. This means that orbitals with lower principal quantum numbers (n) fill before those with higher n values. Within a shell, the subshells fill in the order s, p, d, f.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any one orbital. This maximizes the total spin and results in greater stability. This is often represented using diagrams with arrows representing electrons and separate boxes representing orbitals.
Electron Configurations of the First 20 Elements
Now, let's apply these principles to determine the electron configurations of the first 20 elements. We will represent the electron configuration using the notation: nℓˣ, where 'n' is the principal quantum number, 'ℓ' is the subshell (s, p, d, f), and 'x' is the number of electrons in that subshell.
1. Hydrogen (H): 1s¹
Hydrogen has only one electron, which fills the lowest energy level, the 1s orbital.
2. Helium (He): 1s²
Helium has two electrons, both filling the 1s orbital. This completes the first shell.
3. Lithium (Li): 1s²2s¹
Lithium's third electron begins filling the second shell, specifically the 2s orbital.
4. Beryllium (Be): 1s²2s²
Beryllium's fourth electron completes the 2s orbital.
5. Boron (B): 1s²2s²2p¹
Boron's fifth electron begins filling the 2p subshell.
6. Carbon (C): 1s²2s²2p²
Carbon has two electrons in the 2p subshell, each occupying a separate orbital according to Hund's Rule.
7. Nitrogen (N): 1s²2s²2p³
Nitrogen has three electrons in the 2p subshell, each in a separate orbital.
8. Oxygen (O): 1s²2s²2p⁴
Oxygen's 2p subshell now begins to have paired electrons.
9. Fluorine (F): 1s²2s²2p⁵
Fluorine has only one unpaired electron in the 2p subshell.
10. Neon (Ne): 1s²2s²2p⁶
Neon completes the second shell with a full 2p subshell. This is a noble gas, highly stable due to its complete outermost electron shell.
11. Sodium (Na): 1s²2s²2p⁶3s¹
Sodium's eleventh electron begins filling the third shell, starting with the 3s orbital.
12. Magnesium (Mg): 1s²2s²2p⁶3s²
Magnesium completes the 3s orbital.
13. Aluminum (Al): 1s²2s²2p⁶3s²3p¹
Aluminum starts filling the 3p subshell.
14. Silicon (Si): 1s²2s²2p⁶3s²3p²
15. Phosphorus (P): 1s²2s²2p⁶3s²3p³
16. Sulfur (S): 1s²2s²2p⁶3s²3p⁴
17. Chlorine (Cl): 1s²2s²2p⁶3s²3p⁵
18. Argon (Ar): 1s²2s²2p⁶3s²3p⁶
Argon is another noble gas with a complete outermost shell.
19. Potassium (K): 1s²2s²2p⁶3s²3p⁶4s¹
Potassium's nineteenth electron begins filling the fourth shell, starting with the 4s orbital, illustrating that the 4s subshell has a lower energy level than the 3d subshell.
20. Calcium (Ca): 1s²2s²2p⁶3s²3p⁶4s²
Calcium completes the 4s orbital.
Significance of Electron Configuration: Predicting Chemical Properties
The electron configuration of an element directly influences its chemical properties. The outermost electrons, called valence electrons, are primarily responsible for an element's reactivity. Elements with full outer shells (like the noble gases) are generally unreactive because they are already stable. Elements with incomplete outer shells tend to react in ways that allow them to achieve a full outer shell, either by gaining, losing, or sharing electrons. This drive towards stability underpins the formation of chemical bonds.
For instance, sodium (Na), with its single valence electron in the 3s orbital, readily loses this electron to form a +1 ion, achieving the stable electron configuration of neon. Chlorine (Cl), with seven valence electrons, readily gains one electron to form a -1 ion, also achieving the stable configuration of argon. This exchange of electrons forms an ionic bond between sodium and chlorine, resulting in the formation of sodium chloride (NaCl), common table salt.
Beyond the First 20 Elements: Expanding the Concepts
While this guide focuses on the first 20 elements, the principles of electron configuration extend to all elements in the periodic table. The filling of orbitals follows the Aufbau principle and Hund's rule, although the order of filling can become more complex for heavier elements with more electrons and more energy levels. Understanding the electron configurations of the first 20 elements provides a strong foundation for exploring the complexities of atomic structure and the chemical behavior of all elements. The introduction of the d and f subshells adds another layer of complexity, leading to the fascinating variations in properties we observe across the periodic table. Further exploration into quantum mechanics provides a deeper understanding of the mathematical basis for these rules and the behavior of electrons within atoms.
Conclusion
Mastering the electron configuration of elements is crucial for a deep understanding of chemistry. By comprehending the Aufbau principle, Hund's rule, and the significance of shells, subshells, and orbitals, you gain the ability to predict the chemical properties and reactivity of elements. This knowledge serves as a cornerstone for exploring more advanced concepts in chemistry, laying a solid foundation for future studies in this fascinating field. The first 20 elements provide a perfect starting point for building this essential understanding, offering a manageable introduction to the complexities of atomic structure.
Latest Posts
Latest Posts
-
Indus Valley Civilization Pdf For Class 6
Apr 09, 2025
-
What Is The Prime Factorization For 180
Apr 09, 2025
-
What Is The Least Reactive Metal
Apr 09, 2025
-
How Many Feet In 14 Meters
Apr 09, 2025
-
What Are The Factors Of 43
Apr 09, 2025
Related Post
Thank you for visiting our website which covers about Electron Configuration For First 20 Elements . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.