Does Liquid Have A Definite Volume
Juapaving
Mar 19, 2025 · 5 min read
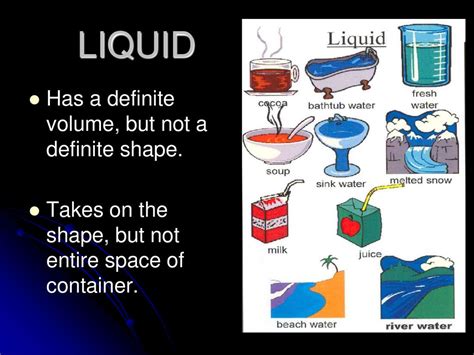
Table of Contents
Does Liquid Have a Definite Volume? Exploring the Properties of Liquids
The question of whether liquids have a definite volume is deceptively simple. While the answer is generally "yes," a deeper understanding requires exploring the unique properties of liquids and the nuances of their behavior under different conditions. This article will delve into the characteristics of liquids, exploring their molecular structure, how they differ from solids and gases, and the factors that can influence their apparent volume.
Understanding the States of Matter
To understand why liquids possess a definite volume, it's crucial to compare them with solids and gases. Each state of matter exhibits distinct properties dictated by the arrangement and interactions of its constituent particles (atoms or molecules).
Solids: Fixed Shape and Volume
Solids possess a definite shape and volume. This is due to the strong intermolecular forces holding their particles in a rigid, closely packed arrangement. The particles vibrate in place but don't move freely, resulting in a fixed structure that maintains its shape and volume regardless of the container. Think of a block of ice – it maintains its shape and volume unless external forces like melting or breaking alter its structure.
Gases: Indefinite Shape and Volume
Gases, on the other hand, have neither a definite shape nor a definite volume. Their particles are far apart and move randomly, experiencing weak intermolecular forces. They readily expand to fill any container they occupy, taking on the container's shape and volume. A balloon filled with air is a perfect example – the air expands to fill the balloon's volume.
Liquids: The In-Between State
Liquids occupy a unique position between solids and gases. They possess a definite volume but an indefinite shape. This is because their particles are closer together than in gases, experiencing stronger intermolecular forces than gases but weaker than solids. These forces restrict the particles from spreading out indefinitely, maintaining a constant volume. However, the particles can move past each other, allowing the liquid to conform to the shape of its container. Water poured into a glass takes on the shape of the glass, but its volume remains the same.
The Molecular Explanation for Definite Volume
The definite volume of liquids stems directly from the balance between intermolecular attractive forces and the kinetic energy of the particles.
Intermolecular Forces: The Glue Holding it Together
The intermolecular forces – forces of attraction between molecules – play a crucial role. These forces, including van der Waals forces, hydrogen bonding, and dipole-dipole interactions, pull the molecules towards each other. The strength of these forces varies depending on the type of liquid. For instance, water molecules have strong hydrogen bonds, resulting in a relatively high surface tension and a more resistant volume.
Kinetic Energy: The Movement that Matters
The kinetic energy of the molecules counteracts the attractive forces. At a given temperature, the molecules possess a certain amount of kinetic energy, causing them to move and vibrate. This movement prevents the molecules from collapsing into a solid-like structure. The balance between these intermolecular attractions and kinetic energy determines the liquid's density and maintains its definite volume.
Factors Influencing Apparent Liquid Volume
While a liquid maintains a definite volume, certain factors can seemingly affect its measured volume. Understanding these nuances is crucial for accurate scientific measurements and practical applications.
Temperature: Thermal Expansion
Temperature significantly impacts the volume of a liquid. As temperature increases, the kinetic energy of the molecules increases, causing them to move further apart. This results in thermal expansion, a slight increase in the liquid's volume. Conversely, as temperature decreases, the volume contracts. This effect is often subtle but must be accounted for in precise measurements, especially for liquids used in highly sensitive instruments or processes.
Pressure: Compressibility
Liquids are generally considered incompressible, meaning their volume doesn't change significantly under pressure. However, under extremely high pressures, some compression does occur. This compressibility is much less pronounced than in gases, where significant volume changes occur even with relatively small pressure changes.
Dissolved Substances: Solutes and Volume
Adding substances to a liquid, like dissolving salt in water, can impact the overall volume. While the volume of the solution might appear to increase slightly, this is largely due to the added volume of the solute. The solvent molecules rearrange to accommodate the solute molecules; the actual volume change is often minor and depends on the properties of both solute and solvent.
Practical Implications of Liquid's Definite Volume
The definite volume of liquids has far-reaching implications across various fields.
Accurate Measurement and Dosage
The consistent volume of liquids is crucial for accurate measurements in numerous scientific, medical, and industrial applications. From precise dispensing of medication to calibrated laboratory experiments, the ability to reliably measure a liquid's volume is paramount.
Fluid Mechanics and Engineering
Understanding the properties of liquids, including their definite volume, is fundamental to fluid mechanics and engineering. Designing pipelines, pumps, and other fluid handling systems relies on the accurate prediction of liquid behavior based on their volume and other properties.
Environmental Studies and Hydrology
In environmental studies and hydrology, understanding the volume of water in rivers, lakes, and oceans is critical for managing water resources, predicting floods, and assessing environmental impacts.
Chemical Reactions and Processes
In chemical reactions, the precise volumes of liquids involved are often crucial for determining reaction stoichiometry and yields.
Conclusion: The Definite Nature of Liquid Volume
In conclusion, while seemingly simple, the question of whether liquids have a definite volume demands a nuanced understanding of their molecular behavior. Liquids do indeed possess a definite volume, a property stemming from the balance between intermolecular attractive forces and the kinetic energy of their molecules. This property, while influenced slightly by temperature and pressure, forms the foundation for numerous scientific, engineering, and practical applications, highlighting the significant role of liquids in our everyday lives and various scientific endeavors. Understanding the intricacies of liquid volume is essential for a variety of disciplines, emphasizing the importance of continuous research and exploration in this field. Further research into the behaviour of liquids under extreme conditions will continue to refine our understanding of this fundamental property of matter.
Latest Posts
Latest Posts
-
What Is The Colour Of Copper Oxide
May 09, 2025
-
Como Se Escribe 460 En Ingles
May 09, 2025
-
Moral Of The Story Of Lion And The Mouse
May 09, 2025
-
A Good Word That Starts With E
May 09, 2025
-
What Part Of A Flower Develops Into A Fruit
May 09, 2025
Related Post
Thank you for visiting our website which covers about Does Liquid Have A Definite Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.