Do Metals Tend To Gain Electrons
Juapaving
Mar 13, 2025 · 5 min read
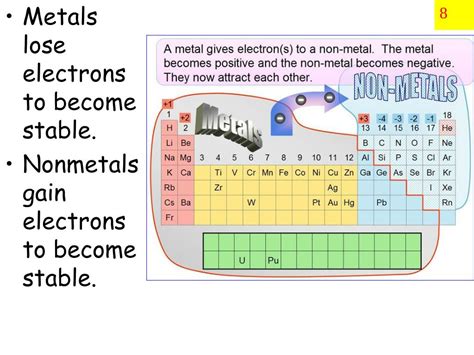
Table of Contents
Do Metals Tend to Gain Electrons? Understanding Metallic Properties and Electron Behavior
The question of whether metals tend to gain electrons is a fundamental concept in chemistry. The simple answer is no, metals generally do not gain electrons. Instead, they tend to lose electrons, a characteristic that defines their metallic nature and influences their chemical behavior and properties. This article will delve deep into the reasons behind this behavior, exploring the electronic structure of metals, their ionization energies, and the resulting formation of cations. We'll also touch upon exceptions to this rule and discuss the implications of this electron behavior in various contexts.
Understanding Electron Configuration and Ionization Energy
The key to understanding why metals lose electrons lies in their electronic configuration. Atoms strive for stability, often achieved by having a full valence electron shell. Metals, typically located on the left side of the periodic table, have relatively few electrons in their outermost shell (valence electrons). These valence electrons are relatively loosely held compared to those in non-metals.
Ionization energy is the energy required to remove an electron from a gaseous atom or ion. Metals generally have low ionization energies. This means it requires relatively little energy to remove an electron from a metal atom. This low ionization energy directly contributes to their tendency to lose electrons rather than gain them. Gaining electrons would require overcoming the repulsive forces between the negatively charged electrons and the existing electrons in the atom, an energetically unfavorable process for metals.
The Role of Effective Nuclear Charge
The effective nuclear charge (Z<sub>eff</sub>) also plays a crucial role. Z<sub>eff</sub> represents the net positive charge experienced by an electron in a multi-electron atom. It's the difference between the nuclear charge (number of protons) and the shielding effect of inner electrons. In metals, the shielding effect is significant, reducing the effective nuclear charge experienced by the valence electrons. This weaker attraction allows the valence electrons to be more easily removed.
Formation of Cations: The Defining Characteristic of Metals
When a metal atom loses electrons, it forms a cation, a positively charged ion. This is a hallmark characteristic of metallic behavior. The number of electrons lost typically corresponds to the number of valence electrons, resulting in a stable electron configuration. For instance, sodium (Na) has one valence electron and readily loses it to form a Na<sup>+</sup> cation with a stable electron configuration similar to neon (Ne).
Examples of Metal Cation Formation
Let's examine some examples to illustrate this:
- Magnesium (Mg): Magnesium has two valence electrons and readily loses them to form Mg<sup>2+</sup>.
- Aluminum (Al): Aluminum has three valence electrons and forms Al<sup>3+</sup>.
- Iron (Fe): Iron can form multiple cations (Fe<sup>2+</sup> and Fe<sup>3+</sup>) depending on the reaction conditions. This variable behavior is due to the complex electronic structure of transition metals.
Exceptions and Complicated Scenarios
While the general rule holds true for most metals, there are exceptions and scenarios that require a more nuanced understanding.
Transition Metals and Variable Oxidation States
Transition metals, located in the middle of the periodic table, often exhibit variable oxidation states. This means they can lose different numbers of electrons depending on the chemical environment. This stems from the complex electronic configuration involving the d orbitals, which allows for multiple possible stable configurations. The oxidation state reflects the number of electrons lost. For example, iron can form Fe<sup>2+</sup> (losing two electrons) or Fe<sup>3+</sup> (losing three electrons).
Organometallic Compounds and Electron Sharing
In organometallic chemistry, metals can form covalent bonds by sharing electrons with organic molecules. In these cases, the metal doesn't strictly lose electrons, but rather participates in a more complex electron sharing arrangement. This demonstrates that the simple "losing electrons" model is an oversimplification for certain chemical interactions.
The Implications of Electron Loss in Metallic Properties
The tendency of metals to lose electrons is responsible for many of their characteristic properties:
- Electrical conductivity: The readily available and mobile valence electrons allow for efficient electrical current conduction.
- Thermal conductivity: The free electrons also facilitate efficient transfer of heat energy.
- Malleability and ductility: The non-directional nature of metallic bonding allows metals to be easily shaped and drawn into wires without fracturing.
- Metallic luster: The interaction of light with the delocalized electrons gives metals their characteristic shiny appearance.
Contrasting Metals with Non-metals
To further emphasize the distinctive nature of metals, let's contrast their electron behavior with that of non-metals. Non-metals, located on the right side of the periodic table, tend to have high ionization energies. This means they require significant energy to remove electrons. Instead of losing electrons, non-metals generally gain electrons to achieve a stable electron configuration. This electron gain results in the formation of anions, negatively charged ions. This fundamental difference in electron behavior is a critical distinction between metallic and non-metallic elements.
Conclusion: A Predominant, but Not Absolute, Trend
In summary, while there are exceptions and complexities, the general trend is clear: metals tend to lose electrons, forming positively charged cations. This fundamental behavior arises from their electronic structure, low ionization energies, and the pursuit of a stable electron configuration. Understanding this principle is crucial for grasping the chemical properties, reactivity, and wide range of applications of metallic elements. The formation of cations is the cornerstone of many chemical reactions involving metals, impacting fields ranging from materials science to biology. While the concept may seem straightforward, exploring the nuances and exceptions illuminates the rich diversity and complexity of chemical behavior. This depth of understanding is critical for advancements in various scientific fields and technological applications that rely on the unique properties of metals.
Latest Posts
Latest Posts
-
Conserving Fuel Is Important Because Burning Fuel
May 09, 2025
-
Red Blood Cells Placed In A Hypotonic Solution Will
May 09, 2025
-
How Do You Write 1 2 As A Percentage
May 09, 2025
-
How Many Times Does Dna Replicate In Mitosis
May 09, 2025
-
How Many Square Feet Is 400 Square Meters
May 09, 2025
Related Post
Thank you for visiting our website which covers about Do Metals Tend To Gain Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.