Do Diastereomers Have Different Physical Properties
Juapaving
Mar 13, 2025 · 5 min read
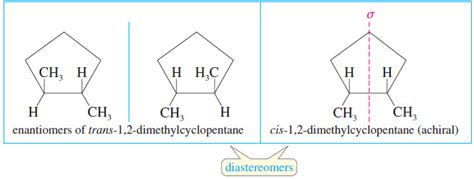
Table of Contents
Do Diastereomers Have Different Physical Properties? A Comprehensive Exploration
Diastereomers, a fascinating class of stereoisomers, hold a significant place in organic chemistry. Understanding their properties, particularly their physical differences, is crucial for various applications, from drug development to materials science. This article delves deep into the question: do diastereomers have different physical properties? The answer, in short, is a resounding yes, and we'll explore why, examining the subtle yet impactful variations that set them apart.
Understanding Diastereomers: A Recap
Before diving into their physical properties, let's briefly revisit the definition of diastereomers. Stereoisomers, in general, are molecules with the same molecular formula and connectivity but different spatial arrangements of atoms. Diastereomers are a subset of stereoisomers that are not mirror images of each other. This crucial distinction differentiates them from enantiomers, which are non-superimposable mirror images.
Diastereomers arise when a molecule possesses two or more chiral centers. A chiral center is a carbon atom bonded to four different groups. The presence of multiple chiral centers allows for various stereoisomeric forms, some of which are diastereomers.
A simple analogy can help visualize this: imagine a pair of gloves. Your left and right gloves are enantiomers – mirror images. Now, imagine a left glove and a left mitten. These are both "left-handed," but they are not mirror images; they are diastereomers.
Why Diastereomers Exhibit Different Physical Properties
The key to understanding the different physical properties of diastereomers lies in their distinct three-dimensional structures. Unlike enantiomers, which have identical physical properties in achiral environments (except for their interaction with plane-polarized light), diastereomers possess different:
-
Melting points: Diastereomers have different melting points because their intermolecular forces (like van der Waals forces, dipole-dipole interactions, and hydrogen bonding) vary depending on their unique spatial arrangements. The way molecules pack together in the solid state is directly influenced by these forces, leading to different melting points.
-
Boiling points: Similarly, boiling points are affected by the strength of intermolecular forces. Diastereomers with different shapes and polarities will exhibit different boiling points. Those with stronger intermolecular interactions require more energy to transition to the gaseous phase.
-
Solubility: Solubility is highly dependent on the molecular shape and polarity of the compound. Because diastereomers possess different shapes and distributions of polar groups, they will exhibit different solubilities in various solvents. A diastereomer might be more soluble in a polar solvent while its diastereomer is more soluble in a nonpolar one.
-
Density: The density of a substance is related to its mass and volume. Different spatial arrangements in diastereomers can result in variations in their overall packing efficiency, leading to differing densities.
-
Optical Rotation: While diastereomers do not exhibit the exact opposite optical rotations like enantiomers, they can still have different optical rotations. The magnitude and direction of rotation are related to their specific three-dimensional structures and the interaction of light with their chiral centers. However, this is less of a defining characteristic than the differences in other physical properties.
-
Chromatographic behavior: Separation techniques like chromatography rely on differences in intermolecular interactions between the analytes and the stationary phase. Diastereomers, with their unique interactions, will have different retention times and elute at different points in a chromatographic separation. This allows for the isolation and purification of individual diastereomers.
Examples Illustrating the Differences
Let's examine a couple of examples to solidify our understanding:
1. Tartaric Acid: Tartaric acid is a classic example showcasing diastereomerism. It has two chiral centers, leading to three stereoisomers: two enantiomers (D- and L-tartaric acid) and one meso compound (meso-tartaric acid). The meso-tartaric acid is a diastereomer of both D- and L-tartaric acid. These three forms exhibit different melting points and solubilities.
2. 2,3-Dibromobutane: This molecule also demonstrates diastereomerism. It has two chiral centers, resulting in four stereoisomers: two pairs of enantiomers and two diastereomers. These diastereomers possess distinct physical properties, allowing for their separation and identification.
Applications Leveraging Diastereomeric Differences
The fact that diastereomers have different physical properties has profound implications in several fields:
-
Drug development: Many pharmaceuticals are chiral molecules, and different diastereomers can exhibit different pharmacological activities. Sometimes, only one diastereomer possesses the desired therapeutic effect, while others may be inactive or even toxic. Understanding and separating diastereomers is therefore crucial for drug development and safety.
-
Material science: The unique physical properties of diastereomers can be exploited to design materials with specific characteristics. For example, variations in melting point, solubility, and density can be used to tailor the properties of polymers and other materials.
-
Chemical analysis: The differences in physical properties make diastereomers readily separable using techniques like chromatography and recrystallization. This allows for the identification and quantification of individual diastereomers in a mixture.
Advanced Concepts and Considerations
-
Conformational Diastereomers: While we’ve focused on configurational diastereomers (those with different arrangements around chiral centers), it's important to note that conformational diastereomers (different conformations with significant energy barriers) also exist. Although these often interconvert readily, they can exhibit slight differences in physical properties under specific conditions.
-
Influence of Solvent: The physical properties of diastereomers can be subtly influenced by the solvent they are dissolved in. Solvent effects can modify intermolecular interactions, leading to slight variations in measured properties.
-
Limitations of Simple Predictions: While we can often predict general trends in physical properties based on structural differences, accurate prediction of specific values (e.g., exact melting point difference) can be complex and may require advanced computational techniques.
Conclusion: The Significance of Diastereomeric Differences
The exploration of diastereomers and their properties reveals a world of subtle yet significant differences in their physical characteristics. Their distinct melting points, boiling points, solubilities, densities, and chromatographic behavior stem directly from their unique three-dimensional structures. These differences are not merely academic curiosities; they hold significant practical implications across diverse scientific disciplines, particularly in drug design, materials science, and chemical analysis. Understanding these differences is essential for researchers and professionals working in these fields. The ability to separate and characterize diastereomers is a critical tool that underpins advancements in various areas of science and technology. As our understanding of stereochemistry continues to evolve, so too will our ability to leverage the unique properties of diastereomers for innovative applications.
Latest Posts
Latest Posts
-
What Are The Rows Called On The Periodic Table
Mar 13, 2025
-
Which Expression Is Equivalent To Sqrt 80
Mar 13, 2025
-
D B S Bank Full Form
Mar 13, 2025
-
Identify The Primary Functions Of Areolar Connective Tissue
Mar 13, 2025
-
What Are All Factors Of 8
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Do Diastereomers Have Different Physical Properties . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
