Do Acids Turn Litmus Paper Blue
Juapaving
Mar 21, 2025 · 6 min read
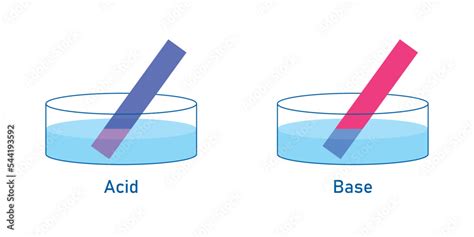
Table of Contents
Do Acids Turn Litmus Paper Blue? Understanding pH and Indicators
The question, "Do acids turn litmus paper blue?" is a common one, and the short answer is no. Acids do not turn litmus paper blue. This seemingly simple question opens the door to a fascinating exploration of chemistry, specifically the concepts of pH, indicators, and how we measure acidity and alkalinity. This comprehensive guide will delve into the intricacies of litmus paper, its interaction with acids and bases, and the broader context of pH measurement.
Understanding pH: The Scale of Acidity and Alkalinity
Before we discuss litmus paper, it's crucial to understand the concept of pH. The pH scale is a logarithmic scale ranging from 0 to 14, representing the concentration of hydrogen ions (H⁺) in a solution. A lower pH indicates a higher concentration of H⁺ ions, signifying a more acidic solution. Conversely, a higher pH indicates a lower concentration of H⁺ ions and a more alkaline (basic) solution.
- pH 0-7: Acidic solutions. The lower the number, the stronger the acid. Examples include stomach acid (around pH 1.5-3.5) and lemon juice (around pH 2).
- pH 7: Neutral solution. Pure water has a pH of 7.
- pH 7-14: Alkaline (basic) solutions. The higher the number, the stronger the base. Examples include baking soda (around pH 9) and household ammonia (around pH 11-12).
Understanding the pH scale is fundamental to comprehending how litmus paper and other pH indicators function.
Litmus Paper: A Natural pH Indicator
Litmus paper is a type of pH indicator, a substance that changes color depending on the pH of the solution it's in contact with. It's derived from a mixture of natural dyes extracted from lichens. These dyes contain various chemical compounds that exhibit different colors at different pH levels. The color change isn't a sudden transition but a gradual shift across a range of pH values.
Key Characteristics of Litmus Paper:
- Widely Available: Litmus paper is readily available in most educational settings and laboratories. It's inexpensive and easy to use, making it a popular tool for basic pH testing.
- Simple to Use: Simply dip a strip of litmus paper into the solution and observe the color change. No specialized equipment is needed.
- Limited Accuracy: Litmus paper provides a qualitative assessment rather than a precise quantitative measurement. It only indicates whether a solution is acidic or basic, not the exact pH value.
- Two Types: Litmus paper comes in two forms: red litmus paper and blue litmus paper. These two forms react differently to acids and bases.
How Litmus Paper Works: The Color Change Mechanism
The color change in litmus paper is due to the change in the chemical structure of the dye molecules in response to the hydrogen ion concentration. In acidic solutions, the dye molecules are protonated (meaning they gain a hydrogen ion), leading to a color change. In basic solutions, the dye molecules are deprotonated (losing a hydrogen ion), resulting in a different color change.
Red Litmus Paper: Red litmus paper turns blue in the presence of a base (alkaline solution). This is because the base deprotonates the dye molecules, causing a color change to blue.
Blue Litmus Paper: Blue litmus paper turns red in the presence of an acid. This is because the acid protonates the dye molecules, causing a color change to red.
This differential reaction is the key to understanding the behavior of litmus paper in relation to acids and bases.
Why Acids Don't Turn Litmus Paper Blue: A Clarification
To reiterate, acids do not turn litmus paper blue. It's the bases that cause the color change from red to blue in red litmus paper. Acids cause the opposite reaction: they turn blue litmus paper red. The misconception might arise from a misunderstanding of the indicator's behavior or a confusion between acids and bases. The color change is a direct consequence of the solution's pH.
Beyond Litmus Paper: Other pH Indicators
Litmus paper is a useful tool for basic pH testing, but it's not the only pH indicator. Many other indicators exist, offering a wider range of pH detection and often more precise measurements. Some examples include:
- Phenolphthalein: This indicator is colorless in acidic solutions and turns pink in basic solutions.
- Methyl Orange: This indicator is red in acidic solutions and yellow in basic solutions.
- Bromothymol Blue: This indicator is yellow in acidic solutions, green in neutral solutions, and blue in basic solutions.
- Universal Indicator: This is a mixture of several indicators that provides a broader color spectrum across the entire pH range, offering a more accurate estimation of pH value.
Each indicator has its own specific pH range where it changes color, making them suitable for different applications.
Applications of Litmus Paper and pH Indicators
The ability to quickly and easily determine the pH of a solution has numerous applications across various fields:
- Chemistry: In laboratories, litmus paper and other pH indicators are essential tools for qualitative and quantitative analysis of solutions.
- Environmental Monitoring: Monitoring the pH of soil, water, and air is crucial in assessing environmental health. Litmus paper can offer a preliminary assessment.
- Agriculture: Soil pH plays a vital role in plant growth. Farmers use pH indicators to adjust soil pH to optimal levels for specific crops.
- Medicine: The pH of bodily fluids, such as blood and urine, is an important indicator of health. pH indicators can help monitor these levels.
- Food and Beverage Industry: The pH of food products affects taste, texture, and preservation. pH indicators ensure quality control and safety.
The simple litmus test, while limited in its precision, is a fundamental step in many of these applications, often serving as a first indication of a solution's acidity or alkalinity.
Practical Experiments with Litmus Paper
To solidify your understanding, consider conducting these simple experiments:
- Testing Household Substances: Collect various household substances (lemon juice, vinegar, baking soda solution, soap solution, etc.) and test their pH using both red and blue litmus paper. Record your observations and compare them to your expectations based on the known acidity or alkalinity of the substances.
- Acid-Base Titration (Simple Demonstration): While a precise titration requires more sophisticated equipment, you can demonstrate the principle by gradually adding a base to an acidic solution (or vice-versa) while testing with litmus paper. Observe the color change as the solution approaches neutrality (pH 7).
Conclusion: The Importance of Understanding pH
The seemingly simple question of whether acids turn litmus paper blue highlights the importance of understanding fundamental concepts in chemistry, like pH and the use of indicators. While acids do not turn litmus paper blue, they do elicit a significant color change in blue litmus paper, turning it red. This reaction, along with the reaction of bases on red litmus paper, provides a quick and easy method for determining the acidity or alkalinity of a solution. The broader understanding of pH and the applications of various indicators extends far beyond the simple litmus test, touching upon many aspects of science, industry, and everyday life. Further exploration of these concepts will enrich your knowledge and appreciation of the chemical world around us.
Latest Posts
Latest Posts
-
Why Is Melting Of Ice Not A Chemical Reaction
Mar 22, 2025
-
What Is The Chemical Formula Of Gold
Mar 22, 2025
-
Who Was The Father Of Renaissance
Mar 22, 2025
-
Is Melting Ice Cream A Physical Change
Mar 22, 2025
-
What Mountain Range Divides Europe From Asia
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Do Acids Turn Litmus Paper Blue . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
